An in-depth understanding of crystal growth mechanisms and kinetics provides guidance for particle size control. Principally, the descriptions of crystal growths are based on the classical Ostwald Ripening (OR) mechanism and the newly discovered Oriented Attachment (OA) mechanism. However, both of the mechanisms cannot describe the fast crystal growth directly from nano- to microcrystals that was observed sometimes.
Prof. LIN Zhang and her collaborators at Fujian Institute of Research on the Structure of Matter(FJIRSM), Chinese Academy of Sciences, have reported an ultrafast growth of SnO2 nanocrystals directly from ∼4 to ∼350 nm in a hydrothermal process, characterized by “either small or large” in the particle size.
An aggregation-induced fast crystal growth mechanism was proposed to explain this novel growth mode. Kinetic analysis, for the first time, indicates that the steep growth from aggregates to bulk crystals is a first-order reaction with respect to the content of the aggregated nanoparticles.
SAXS data support that the onset of the fast growth is related to an increase in the aggregation degree of the aggregates. The finding in this work provides new threads for syntheses of novel nanomaterials that may possess properties not readily obtained via conventional crystal growth routes (J. Am. Chem. Soc.2012, 134, 16228).
Previously, researchers in Prof. LIN Zhang’s group have developed a strategy to recycle SnO2 nano-wires from tinplate electroplating sludge. By a selective control of the crystallization and growth of SnO2 with an addition of NaOH as a mineralizer, the amorphous Sn compound fast transformed into acid-insoluble SnO2 nanowires. Valuable SnO2 nanowires thus were obtained via a subsequent acid treatment. The strategy could have a potential ability to the recycling of valuable metals from industrial sludge in other areas(J. Hazard. Mater.2012, 211, 41; Physical Chemistry Chemical Physics, 2009,11, 8516).
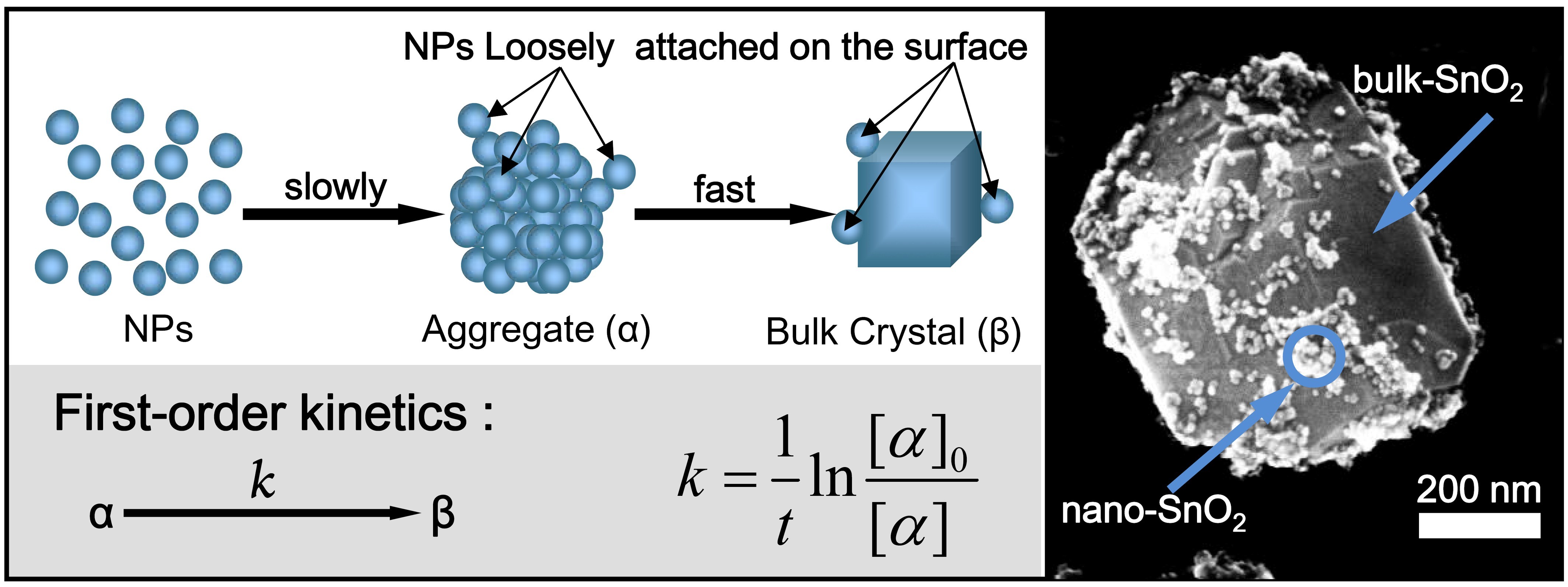
Schematic illustration of aggregation-induced fast crystal growth model
(Image by Prof. LIN Zhang’s group)
Contact:
Prof. LIN Zhang
Fujian Institute of Research on the Structure of Matter
Chinese Academy of Sciences
Email: zlin@fjirsm.ac.cn