Blood flows continuously throughout body for lifetime, but quickly shuts off to prevent spills when one gets a cut or injury. Clotting of blood is healthy and lifesaving when it stop bleeding, but can also form under pathological condition or when blood does not flow properly. Abnormal blood clotting, or thrombosis, causes a stroke, heart attack or other serious medical problems, and is a major contributor to disability and mortality. Thrombosis is estimated to be the cause of one in four deaths worldwide.
To date, the most effective clot lysis therapy (thrombolytic therapy) is to boost up an internal clot lysis enzyme, tissue-type plasminogen activator (tPA) by infusing recombinant tPA (rtPA) into blood. This therapy is FDA-approved and rtPA is the most effective thrombolytic drug for ischemic strokes, myocardial infarction and pulmonary embolism.
High dose of recombinant tPA (up to 100 mg per 50 kg) is typically needed in clinical applications, due to, in part, the rapid inactivation of recombinant tPA by endogenous physiological inhibitors (plasminogen activators inhibitor 1, PAI-1). High dosage of tPA leads to dangerous side effects, e.g., intra-cranial hemorrhage and neurotoxicity, which can be fatal. Engineering on tPA to reduce its inhibition by PAI-1 without compromising its thrombolytic effect is a continuous effort.
A team of scientists led by Prof. HUANG Mingdong from Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, determined the crystal structure of rtPA in complex with its endogenous inhibitor (PAI-1) at 3.16 Å. This structure presents precise details, with atomic resolution, on how the tPA is inhibited by PAI-1.
In this structure, the RCL of PAI-1 serves as a bait to attract the rtPA onto the top of PAI-1 molecule, forming a large interface of 1,202 Å2. Protein engineering of tPA by mutations based on this structure can maximize rtPA resistance to inhibition by PAI-1, hence prolonging the half life of rtPA in vivo. This long-sought structure also explains the PAI-1-resisting property of a third generation of rtPA (Tenecteplase).
Therefore, this work offers avenue to develop a newer generation of clot bustering agent with higher potency by increasing its half life in blood and by reducing drug dose needed in the therapy, and thus reducing the bleeding side effect.
This work is published recently in J. Biol. Chem. (J. Biol. Chem.2015 290: 25795-25804) as a research article entitled "Crystal Structure of the Michaelis Complex between Tissue-type Plasminogen Activator and Plasminogen Activators Inhibitor-1".
This study was funded by the National Natural Science Foundation of China (31170707, 31370737), and CAS/SFEA International Partnership Program for Creative Research Teams.
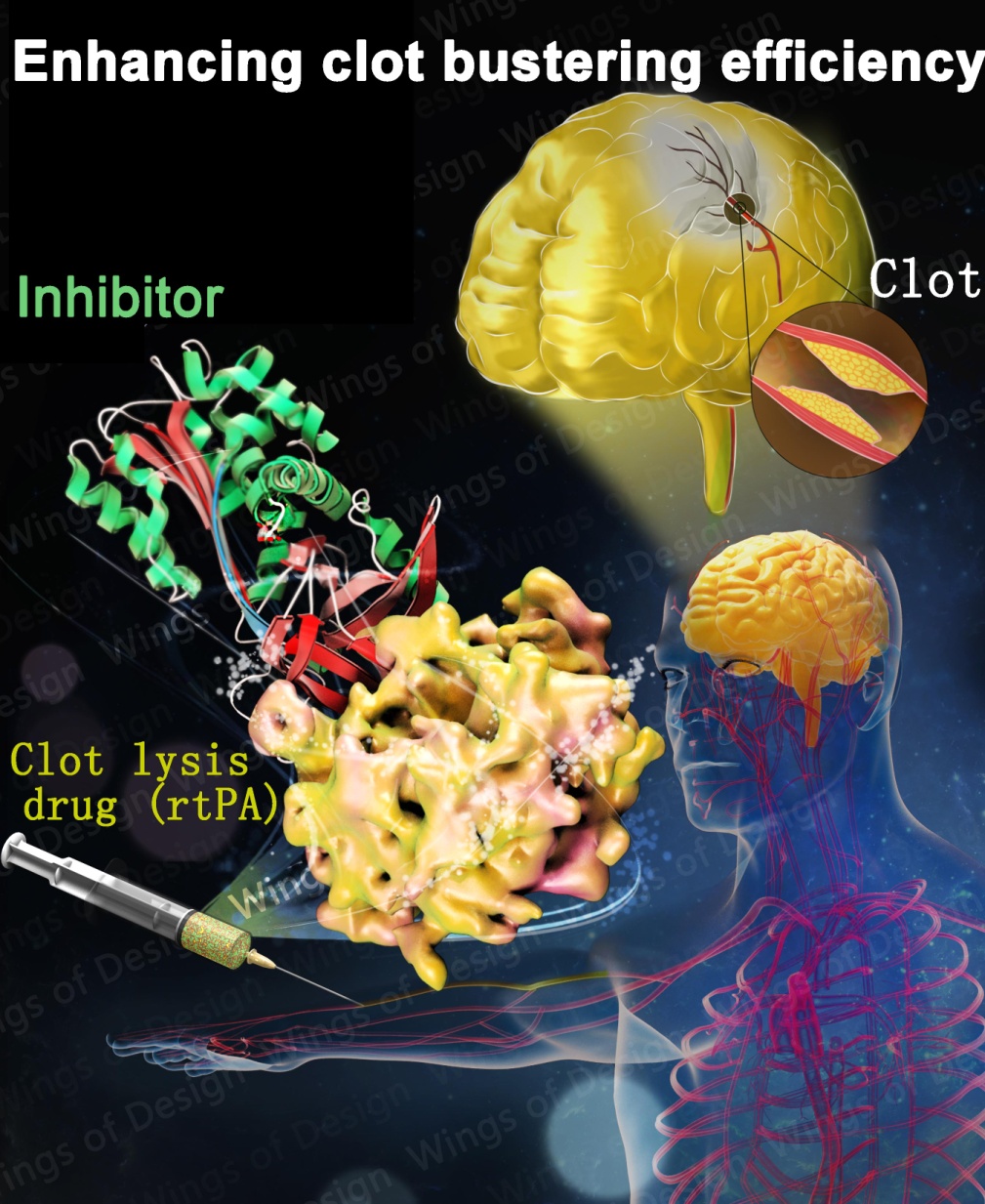
Illustration of a structural basis to design newer thrombolytics. (Image provided by Prof. HUANG Mingdong's group)
Contact:
Mingdong Hunag, Ph.D., Principal Investigator
Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou 350002, China
Tel: 86-591-83704996;
Fax: 86-591-83723096;
E-mail: mhuang@fjirsm.ac.cn