Site selectivity control is an outstanding challenge in the research of direct functionalization of inert C–H bonds. Although several chelation-assisted meta-C–H functionalizations of electron-rich arenes were reported, chelation-assisted meta-C–H functionalization of electron-poor arenes such as benzoic acid derivatives remains a formidable challenge.
In a recent study, a team of scientists led by Prof. LI Gang at Fujian Institute of Research on the Structure of Matter(FJIRSM), Chinese Academy of Sciences(CAS), reported a general protocol for meta-C–H olefination and acetoxylation of benzoic acid derivatives under mild conditions, by utilizing a nitrile-based sulfonamide directing group. The results entitled “Pd(II)-catalysedmeta-C–H functionalizations of benzoic acid derivatives” has been published in Nature Communications.
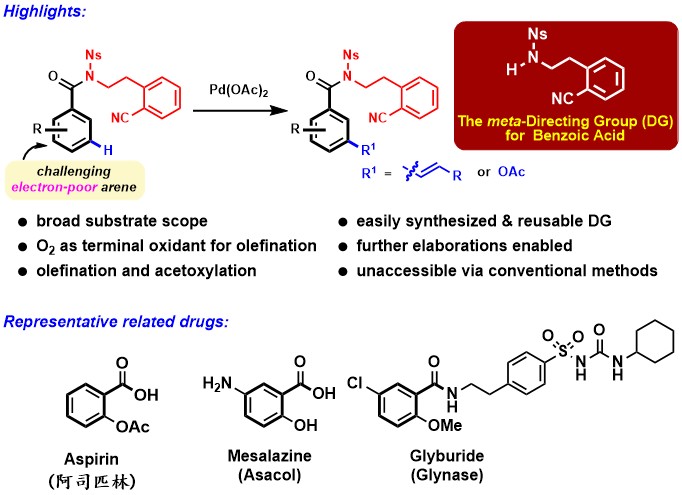
Pd(II)-catalyzed meta-C–H functionalizations of benzoic acid derivatives(Image by Prof. LI's group).
Benzoic acids are highly important structural motifs in drug molecules and natural products. Selective C–H bond functionalization of benzoic acids will provide synthetically useful tools for step-economical organic synthesis. Despite that the direct ortho-C–H functionalizations of benzoic acids have been intensely studied, the activation of meta-C–H bond of benzoic acids or their derivatives in a general manner via transition-metal catalysis has been largely unsuccessful, partially due to the electron-poor property of benzoic acids.
Base on their previous research on the Pd(II)-catalyzed remote regiodivergentortho- andmeta-C–H functionalizations of phenylethylamines that are electron-rich arenes, the researchers developed a new nitrile-based sulfonamide directing group for meta-C–H activation of benzoic acid derivatives that are electron-poor arenes. A broad range of benzoic acid derivatives were meta-selectively olefinated using environmentally benign molecular oxygen as the terminal oxidant. The meta-C–H acetoxylation also occurred smoothly and the corresponding products enabled the access to five synthetically useful classes of substituents at the meta-position of benzoic acid derivatives. This research provides new perspectives for the challenging meta-C–H functionalizations of electron-poor arenes. It is expected that this method will soon find its applications in drug discovery and the synthesis of functional molecules.