Dynamics of water molecules surrounding the hydrated ions affects many natural phenomena including protein processes and charge transfer in the aqueous rechargeable ion batteries. In the concentrated solutions, a long standing puzzle is that all electrolytes retard water rotation regardless of whether they weaken or strengthen water hydrogen bonding network.Recently, Prof. ZHUANG Wei and his colleagues at Fujian Institute of Research on the Structure of Matters, Chinese Academy of Sciences, investigated this issue theoretically. The results were published online in Proc. Nat. Acad. Sci. USA.
A commonly accepted intuitive expectation, proposed by Laage and Jungwirth, is that the altered water hydrogen bond switching behavior around the ions leads to this general retardation. Neutron scattering measurements reveal that the ions enhance the probability to find water molecules which act as the hydrogen bond acceptors in an interstitial position between the first and second hydration shells in pure water.
Some water molecules adjacent to the ions therefore stride over a markedly shorter distance during the hydrogen bond switching, which decelerates the water diffusion and consequently the overall water rotation. This intuitive picture implies a local involvement of ion effects on the water molecules only in its immediate vicinity.
In this study of water reorientational motion in different ionic solutions, massive amount of molecular dynamics simulations was carried out to generate the statistically comprehensive ensemble of water hydrogen bond switching events.
Based on the simulation result, analysis using a rotational markov chain model and a continuous time random walk coarse graining method indicates that the deceleration is in fact largely due to the coupling of slow, collective component of water rotation with the motion of sizable ion clusters in the concentrated solutions.
This finding is at variance with the aforementioned intuitive expectation that the deceleration is caused by the change in fast single molecular water hydrogen bond switching adjacent to the ions.
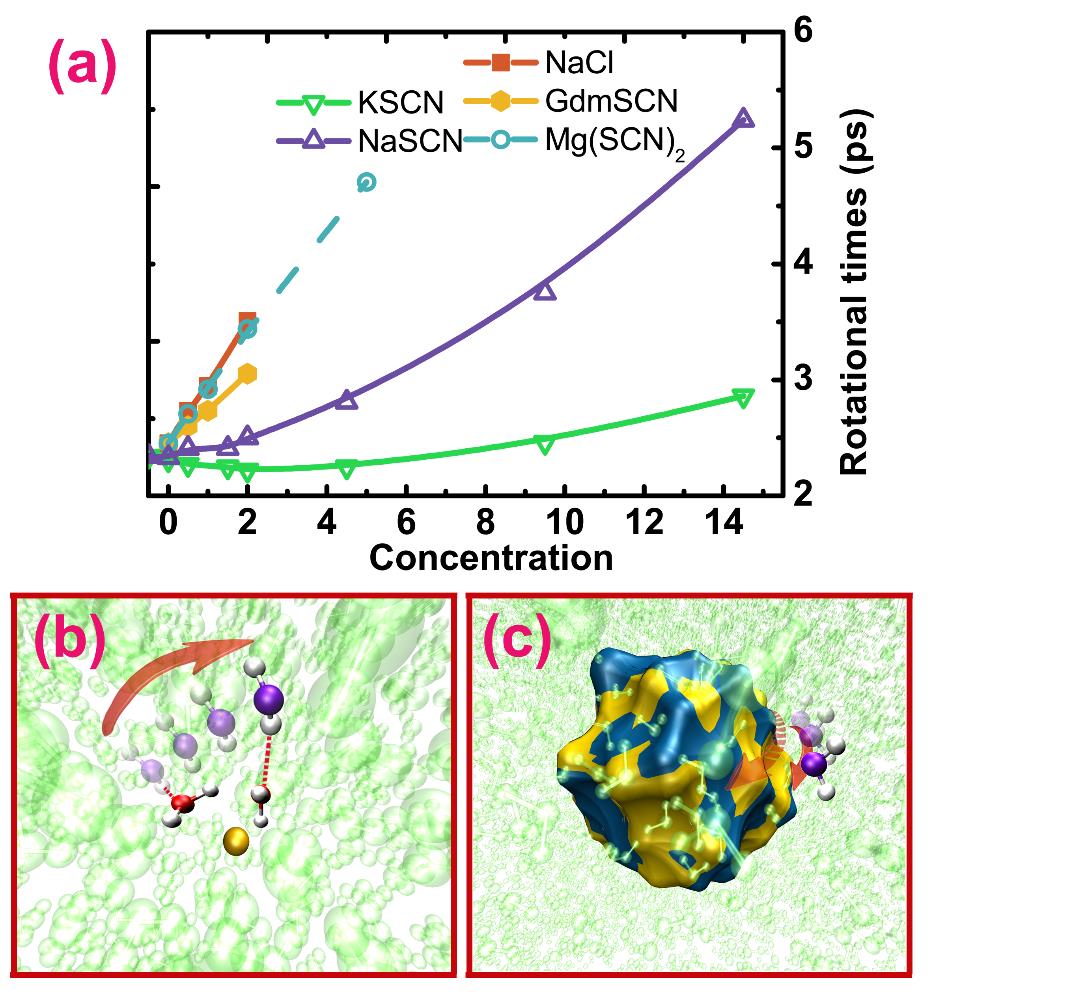
Fig.1. (a): Rotational times of water in various aqueous ionic solutions at different concentrations. (b) and (c): schematic representations of two possible molecular mechanisms leading to the deceleration of water rotation in the concentrated ionic solutions.(Image by Prof. ZHUANG's group)
Contact:
Prof. ZHUANG Wei
Fujian Institute of Research on the Structure of Matter
Chinese Academy of Sciences
Email: wzhuang@fjirsm..ac.cn