With the development of nuclear energy, problems related to the nuclear fuel cycle inevitably arise, such as the effective disposal of nuclear wastes. In nuclear wastes, uranium (U) is a highly toxic radionuclide with carcinogenicity, while 137Cs and 90Sr have a long half-life and biological toxicity. It is very important to develop new materials that can capture the [UO2]2+, Cs+ and Sr2+ ions efficiently and selectively.
In a recent study published in J. Am. Chem. Soc., the research group led by Prof. HUANG Xiaoying and Prof. FENG Meiling at Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, reported two new gallium thioantimonates, namely [Me2NH2]2[Ga2Sb2S7]·H2O (FJSM-GAS-1) and Et2NH2]2[Ga2Sb2S7]·H2O (FJSM-GAS-2), which could quickly and selectively remove the [UO2]2+, Cs+ and Sr2+ ions from solutions.
In this study, the maximum uranium, cesium and strontium ion exchange capacities for FJSM-GAS-1 are 196 mg/g, 164 mg/g, and 80 mg/g, respectively. While the uranium ion exchange capacity for FJSM-GAS-2 is 143 mg/g. Such capacity difference can be attributed to the different cations encapsulated in between the anionic layers of FJSM-GAS-1 and FJSM-GAS-2.
Researchers found that both FJSM-GAS-1 and FJSM-GAS-2 exhibit really rapid ion exchange kinetics, that is, they could complete the ion exchange within 5 minutes and 15 minutes, respectively.
Moreover, both FJSM-GAS-1 and FJSM-GAS-2 could maintain the ion exchange ability and excellent selectivity for [UO2]2+ ion, even in the pH range of 2.9-10.5 and with the existence of Na+, Ca2+ and HCO3- in large quantity. The KdU of FJSM-GAS-1 could reach 6.06 × 10 6 mL/g, which is the highest value among that of the reported U adsorbents.
They showed that the [UO2]2+-laden products of FJSM-GAS-1 and FJSM-GAS-2 could be eluted in a simple and inexpensive way to recover uranium.
The afore-mentioned advantages, combined with the easy synthesis and excellent radiation resistances of β and γ irradiation make FJSM-GAS-1 and FJSM-GAS-2 promising candidates as the materials for capturing radioactive [UO2]2+, Cs+ and Sr2+ from nuclear waste solutions.
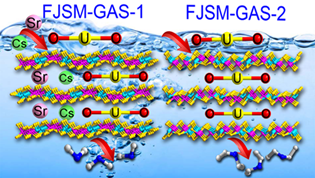
Schematic presentation of the [UO2]2+ , Cs+ and Sr2+ ion exchanges by FJSM-GAS-1 and FJSM-GAS-2 (Image by Prof. HUANG’s Group).
Contact:
Prof. FENG Meiling
Fujian Institute of Research on the Structure of Matter
Chinese Academy of Sciences
Email: fml@fjirsm.ac.cn