Imines and enamines are ubiquitously encountered intermediates in organic synthesis.
The imines containing α-hydrogens stay in equilibrium with their enamine tautomers. This imine-enamine tautomerism is the nitrogen analog to keto-enol tautomerism but with higher reactivity. This feature makes the tautomerizable imine resemble the reactivity of enamine in organic transformations through its enamine tautomer. However, the exact difference in reactivity between the imine-derived enamine tautomer and the real enamine remains unknown.
In a study published in Nat. Commun., the research groups of Prof. SU Weiping and Prof. ZHUANG Wei at Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, developed a unique model reaction pattern that could discriminate this subtle discrepancy, which afforded molecular diversity depending on the chemistry of imine and enamine and offered tremendous insight into the structure-reactivity relationship.
For the oxidation reaction with 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO), the mechanistic studies revealed that the NH-containing enamine kinetically favors α-radical formation by TEMPO-mediated hydrogen atom abstraction from NH moiety and then 1,4-elemination to generate α-amino enones,whereas, enamine in lack of NH part prefers α-radical formation and subsequent consecutive β-elimination of TEMPOH to deliver arylamines.
Therefore, due to imine-enamine tautomerization, the α-hydrogen-containing imine displays distinctly different in regioselectivity and chemoselectivity from the enamine lacking NH moiety.
This protocol offers a simple platform to combine the chemistry of imine and enamine together and to show their reactivity differences through distinctive product distribution under nearly identical reactions conditions.
The underlying mechanism would provide valuable clues to enlighten more diverse reaction patterns based on the reactivity difference of tautomerizable imines and enamines.
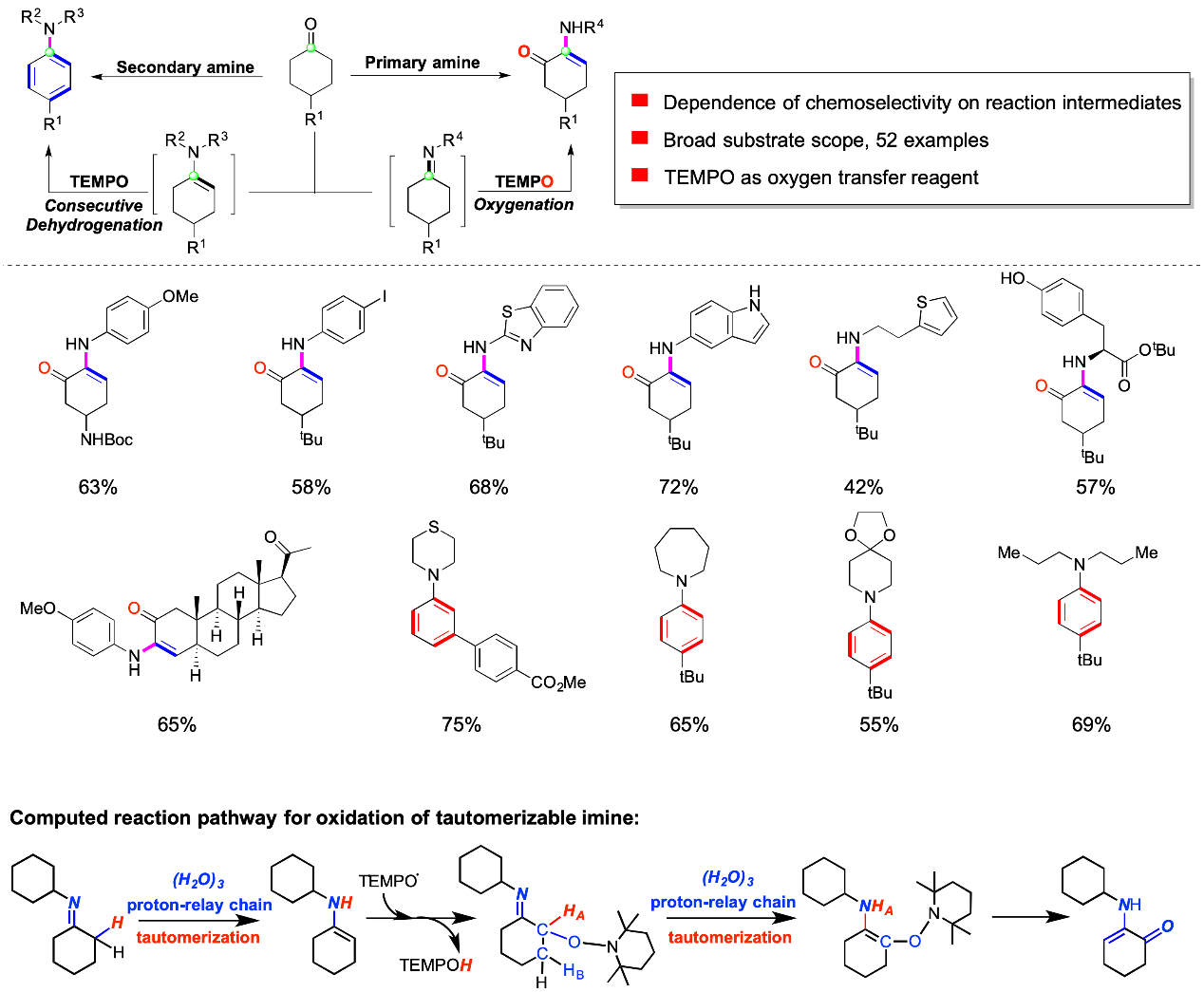
Schematic representation of the dissimilarity in reactivity between enamine and imine.(Image by Prof. SU’s Group)
Contact:
Prof. SU Weiping
Fujian Institute of Research on the Structure of Matter
Chinese Academy of Sciences
Email: wpsu@fjirsm.ac.cn