The use of solar energy to drive challenging organic synthesis has been generating interest due to its environmental friendliness and sustainability.
The transfer pathway and lifetime of photogenerated electron-hole pairs are vital to the efficiency and selectivity of photocatalysis. It has become a research trend that coupling two value-added reactions into one system because both photogenerated e- and h+ exhibit outstanding reducing and oxidizing peculiarity, respectively.
In a study published in Journal of Catalysis, a research group led by Prof. CAO Rong from Fujian Institute of Research on the Structure of Matter (FJIRSM) of Chinese Academy of Sciences reported a coupled system, in which electrons and holes are fully utilized for nitrobenzene photoreduction coupled with alcohols photooxidation.
Researchers found that the hydrogen-bonding interaction between the hydroxyl group of benzyl alcohol and the bridging oxygen atom weakened the interaction between the Ti6c and O2c atoms when the benzyl alcohol adsorbed on the TiO2 surface.
Experimental and theoretical results together reveal that oxygen vacancies (OVs) in situ generated through surface complexation, which broaden the light absorption range and act as a bridge to enable the transfer of the photoinduced electrons.
They unveiled the first example of a coupled system, where the photoexcited electrons and holes are directly and simultaneously utilized for reduction of nitrobenzene and selective oxidation of benzyl alcohol by unmodified TiO2.
The chemisorption on the TiO2 surface decreases the oxidation potential of benzyl alcohol and causes an upward shift in its highest occupied molecular orbital (HOMO), which facilitates the half oxidation reaction of benzyl alcohol to benzaldehyde. Simultaneously, rather than recombine with their counterpart holes, efficient electron transport may occur from the conduction band of TiO2 to nitrobenzene for the other half reduction reaction.
This study highlights a crucial role that heterogeneous surfaces may play in enabling photocatalytic organic transformations. The deeper understanding of the reaction mechanisms also provides a new perspective of utilizing the chemisorption between the reactant and catalyst to achieve a defect engineering strategy for synergetic photocatalysis.
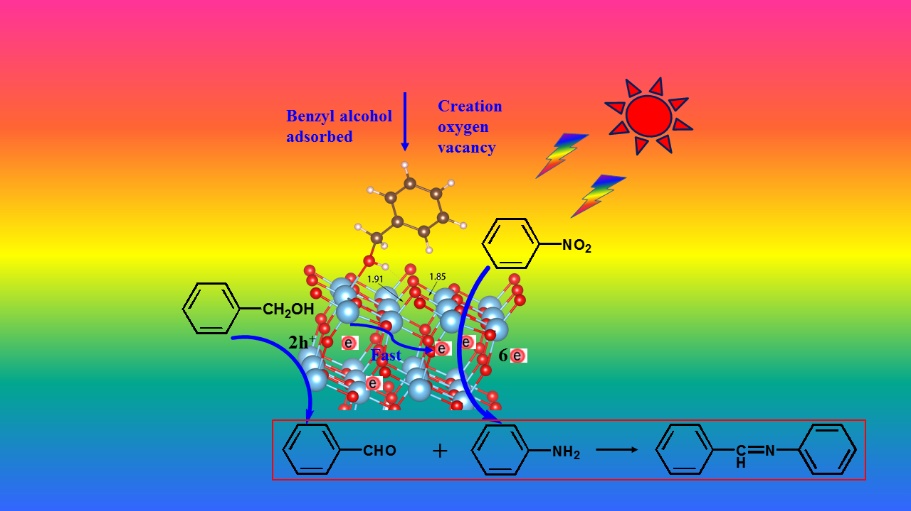
Schematic illustration of the oxygen-vacancy-mediated photocatalytic mechanism of benzyl alcohol and nitrobenzene under visible light catalyzed by TiO2. (Image by Prof. CAO’s group)
Contact:
Prof. CAO Rong
Fujian Institute of Research on the Structure of Matter
Chinese Academy of Sciences
E-mail: rcao@fjirsm.ac.cn