Learning from the extensive hydrogen bonding existing in biological systems, chemists were inspired to use hydrogen bonding to construct porous frameworks, termed as hydrogenbonded organic frameworks (HOFs) or supramolecular-organic frameworks (SOFs). This kind of material has many unique advantages including high crystallinity, large surface area, mild synthetic conditions, solvent processability and show great potential in many applications. However, few structure-property correlations have been explored in this field.
In order to explore the self-assembly rules and realizing the rational design of HOF structures, a research team led by Prof. LIU Tianfu from Fujian Institute of Research on the Structure of Matter of the Chinese Academy of Sciences (FJIRSM, CAS), selected a planar molecule H3TATB and non-planar molecular H3BTB with the same hydrogen bond interaction but slightly different molecular backbone geometry for self-assembly study. This study was published in Journal of the American Chemical Society.
The researchers found that planar H3TATB ligands are interconnected through double hydrogen bonds to form regular hexagonal honeycomb nets (hcb), which are interwoven simultaneously to form extremely complicated two-dimensional (2D) layer structures.
The occurrence of wave-like layers in all these structures was reminiscent of the corrugations observed on suspended or supported graphene sheets, that is, the ultra-thin layer become thermodynamically unstable after exceeding certain dimension, unless the layer structure being perturbed in the third direction or constituting an inherent part of a three-dimensional (3D) system.
Therefore, the researchers speculate that the undulations coupled with parallel polycatenation stabilize the ultra-thin planar fragments formed during self-assembly process. This speculation is supported by the observation that the supernatant solution contains many lamellae when examined by AFM and SEM analyses during the crystallization process.
Meanwhile, undulated single layer structures were also found in other materials based on planar ligands. In contrast, self-assembly of the very similar ligand H3BTB, bearing the same hydrogen-bonding sites but nonplanar backbone, resulted in a flat monolayer, demonstrating that a slight torsion of ligand produces overwhelming structure change.
This structure change delivers PFC-11 and PFC-12 with unique stepwise adsorption behaviors under a certain pressure originating from the movement between mutually interwoven hexagonal networks.
With the excellent stability and framework flexibility, PFC-11 and 12 present promising candidates for carbon dioxide and volatile organic compound adsorption with preferential selectivity toward π-conjugated molecules such as benzene and toluene.
The study helps to understand the self-assembly behaviors of HOFs and sheds light on the rational design of HOF materials for practical applications.
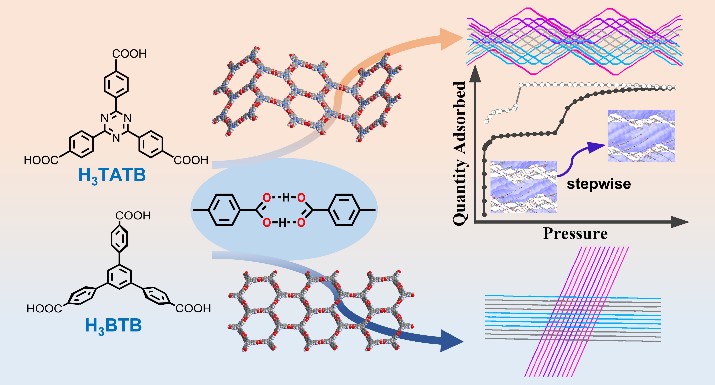
Representation of the chemical structure, the obtained hexagonal honeycomb monolayer and polycatenated networks constructed by H3TATB (upper panel) and H3BTB (lower panel) (Image by LI Yulin)
Contact:
Prof. LIU Tianfu
Fujian Institute of Research on the Structure of Matter
Chinese Academy of Sciences
Email: tfliu@fjirsm.ac.cn