Nonlinear optical (NLO) crystals are the key materials for laser science and technology because they can expand the frequency range of common laser sources. For crystal materials, the functional motif (unit or group) has been used in the literature to describe the “materials gene” which determines a specific material property. Currently, structural groups based on chemical stability are widely used to refer to a functional motif.
However, theoretically, the partitioning method of an extended structure depends closely on its physical property. There are two challenging problems of fundamental importance remained unsolved to date. One is the lack of a quantitative model to guide the partition of an extended structure into its building atomic groups, the other involves the partition or mapping of a physical property onto various atomic groups. Due to the lack of a structural partitioning scheme according to the physical properties, it is impossible to obtain the correct functional motif controlling the specific physical property of materials.
In a study published in Phys. Chem. Chem. Phys., the research group led by Prof. DENG Shuiquan from FJIRSM of the Chinese Academy of Sciences (CAS) developed the general principles for partitioning a structure and its property in terms of a set of atoms and bonds by analyzing the differential cross-sections of neutron and X-ray scattering phenomena, and propose the procedures with which to partition an extended structure and its property.
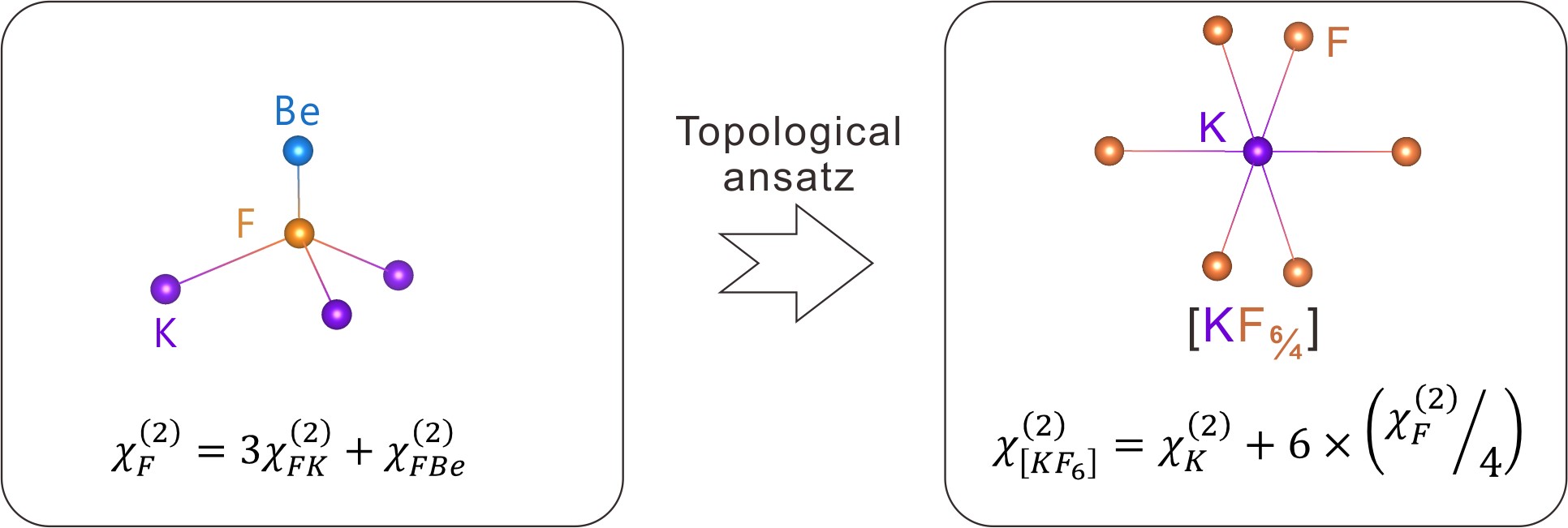
The researchers used the developed principles to partition the structure and the second harmonic generation (SHG) response of KBe2BO3F2 (KBBF), an important deep-ultraviolet NLO crystal.
They calculated the contribution of an atomic group by summing the contributions of the center atom and those of its ligands by partitioning the contribution of an anion equally to all the atomic groups it belongs to, based on the results from atom response theory analysis.
The group contributions for the cation centered groups of KBBF show that the ionic bond group [KF6] or [BeO3F] (total contribution ~81.6%) contributes much more strongly to the SHG response than does the covalent bond group [BO3] (i.e., ~18.4%). Although the ionic bond group [KF6] and the covalent bond group [BO3] are nonpolar, the BeO3F group is polar. Therefore, it is the nonpolar ionic bond group [KF6] that contributes most strongly to the SHG response.
These results demonstrate that the SHG response does not depend on either the dipole moment of the atomic groups or the static polarization of a crystal. A remarkable finding is that the SHG response of the ionic bond groups far surpasses that of the covalent anionic groups.
This study provides significant insight into physical chemistry and opens up a new approach for searching for new materials for SHG.
Partitioning analysis of KBBF (Image by Prof. DENG’s Group)
Contact:
Prof. DENG Shuiquan
Fujian Institute of Research on the Structure of Matter
Chinese Academy of Sciences
Email: sdeng@fjirsm.ac.cn