The umpolung strategies which use D2O as the deuterium source have many advantages in price, supply, and safety to synthesize α-deuterated alcohols in large quantities. However, the umpolung strategies have rarely been utilized in the reductive deuteration of ketones because these methods reported previously would require a large excess amount of D2O or CD3OD (typically more than 100 equiv.).
In a study published in Organic Letters, the research group led by Prof. BAO Hongli from Fujian Institute of Research on the Structure of Matter (FJIRSM) of the Chinese Academy of Sciences, have developed a new and practical umpolung strategy for the reductive deuteration of ketones.
The umpolung strategy in organic chemistry is the chemical modification of a functional group with the aim of the reversal of polarity of that group. Normally, the electricity of the carbon on the ketone group is positive. In this study, the electricity of the carbon on the ketone group reverses to negative by using an Mg/BrCH2CH2Br system. Then, the carbon with negative electricity will react with the positive deuterium in the deuterium water to give α-deuterated alcohols.
The researchers optimized the reaction conditions and the reactions afforded α-deuterated alcohols in yields as high as 88% and deuterium incorporation in excess of 98%. Only 1.5 equiv. of D2O is required for the deuteration while alternative methods need a large excess amount of D2O or CD3OD.
Meanwhile, the researchers investigated the scope of substituted benzophenones. In general, the reactions with variously substituted benzophenones as substates will give the desired products in good yields and with >96% deuterium incorporation. Substrates with a five-, six-, or seven-membered ring can be transformed into the corresponding products in 57-88% yields and with up to 97% deuteration incorporation. The reactions also underwent smoothly with pyridines, affording the target products in 61%-82% yields.
To explore the synthetic potential of this method, the researchers carried out direct reductive deuteration of the drug molecules and synthesis of various deuterated drug molecules. Fenofibrate which is a drug for the treatment of hyperlipidemia and hypertriglyceridemia can be selectively reduced to the corresponding deuterated alcohols.
Besides, the researchers synthesized the deuterated diphenhydramine and buclizine by the current reductive deuteration method. They also synthesized the deuterated (benzhydrylsulfanyl)acetic acid, which can be used as the precursor for the synthesis of deuterated modafinil and deuterated adrafinil.
This study develops a practical method for the reductive deuteration of carbonyl compounds to α-deuterated alcohols with excellent deuterium incorporation. This method features mild reaction conditions, good substrate scope and excellent functional group tolerance. The researchers demonstrated the synthetic value of this method by the easy access to deuterated drugs or drug derivatives.
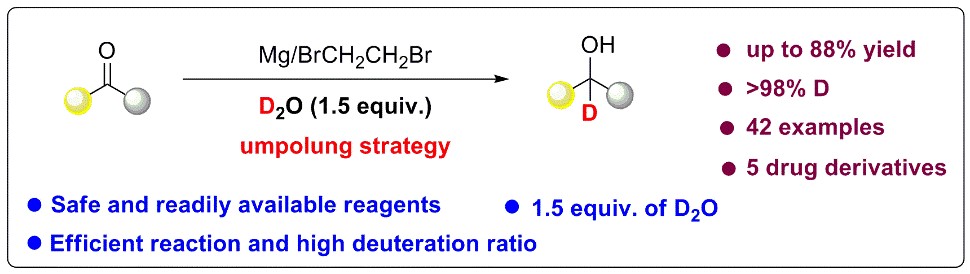
Reductive deuteration of ketones using a Mg/BrCH2CH2Br/D2O system (Image by Prof. BAO’s group)
Contact:
Prof. BAO Hongli
Fujian Institute of Research on the Structure of Matter
Chinese Academy of Sciences
E-mail: hlbao@fjirsm.ac.cn