Hydrogen peroxide (H2O2), as an important industrial chemical, plays a key role in papermaking, textile, environmental management, renewable energy conversion and other fields. In recent years, electrochemical methods in fuel cells have provided an attractive way to produce hydrogen peroxide on site. Carbon-based materials are considered to be promising catalysts for the electrochemical generation of H2O2 due to their low cost, good electrical conductivity, and excellent stability. But the activity of carbon based electrocatalysts in neutral electrolytes is still very low and needs to be improved.
In a study published inACS catalysis, the research group led by Prof. CHAI Guoliang of Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences reported the preparation of mesoporous carbon hollow spheres with different pore sizes by changing the proportion of template precursors.
The researchers used tetrapropyl orthosilicate (TPOS) or tetraethyl orthosilicate (TEOS) as template precursors, and resorcinol and formaldehyde as raw materials to prepare hollow carbon spheres. By changing the ratio of TPOS and TEOS, they obtained the carbon spheres with different pore size and oxygen contents. According to the TPOS:TEOS ratio (silicon precursors), the catalysts are defined as MCHS-TPOS, MCHS-9:1, and MCHS-TEOS. They used electrochemical tests to explore the catalytic performance of different carbon spheres, and synthesized the carbon spheres with different precursor ratios under the same conditions.
The results showed that the selectivity of MCHS-9:1 for hydrogen peroxide formation in 0.1M phosphate buffer solution (pH = 8) is 99.9% (at a potential of 0.57 V), and the selectivity is more than 90% in a wide potential range (0.35 - 0.62 V). This indicates that the mesoporous carbon hollow spheres are a promising electrocatalyst for in-situ preparation of H2O2.
Through a series of characterization methods, the researchers found that the difference of electrochemical performance of different carbon spheres is due to the difference of pore size and oxygen contents. Mesoporous carbon spheres have higher activity than microporous carbon spheres, and C-O group in the oxygen functional groups of carbon spheres is more conducive to improve the selectivity of H2O2.
In order to further verify the effect of oxygen functional groups on the electrochemical performance of MCHS-9:1, the researchers used the hydrogen reduction method to remove oxygen functional groups (R-MCHS-9:1). The content of oxygen decreased from 17.87% to 6.67%. The H2O2 selectivity of R-MCHS-9:1 decreased from 92.3% for MCHS-9: 1 to 56.11% at a potential of 0.4 V vs RHE.
The types of oxygen functional groups in carbon materials have a great influence on the oxygen reduction performance. To further study the formation mechanism of H2O2, the researchers used the Density Functional Theory (DFT) to simulate. The results showed that the catalytic configuration of mixed ketone oxygen and COOH(2COOH2k-O)plays an important role in the catalytic formation of H2O2 in neutral electrolyte. The samples related to C-O group have the best performance in generating H2O2 in alkaline and neutral electrolytes.
The study helps to better understand carbon-based catalysts for H2O2 production and their rational design.
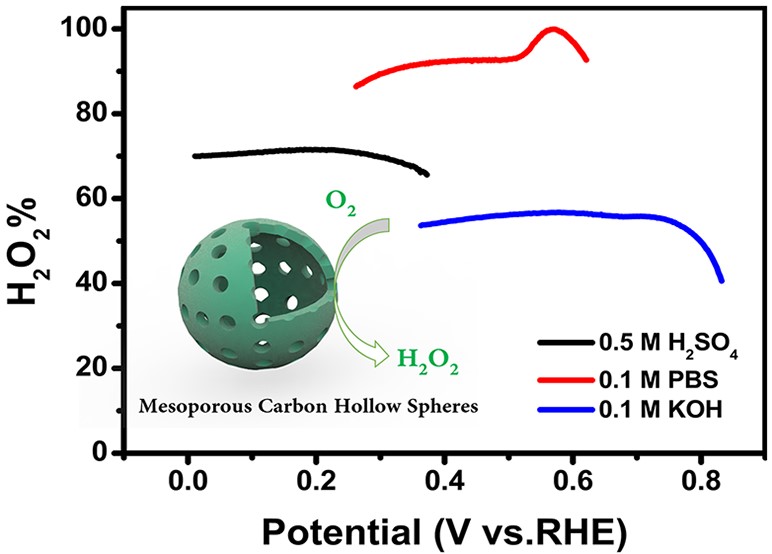
Mesoporous Carbon Hollow Spheres as Efficient Electrocatalysts for Oxygen Reduction to Hydrogen Peroxide in Neutral Electrolytes (Image by Prof. CHAI’s group)
Contact:
Prof. CHAI Guoliang
Fujian Institute of Research on the Structure of Matter
Chinese Academy of Sciences
Email: g.chai@fjirsm.ac.cn