Porous organic cages (POCs) are discrete, covalently linked molecules with intrinsic cavities. The porous nature of POCs enabled them to hold great promise as nanoscale reaction vessels for catalysis, hosts for different guest molecules, adsorbents for gas storage and separation.
POCs materials exhibit solvatomorphs via altering their crystallographic packing in the solid state, but investigating real gas mixture separation by porous materials with such a behavior is still very rare.
In a study published in ACSAppl. Mater. Interfaces , the research group led by Prof. YUAN Daqiang from Fujian Institute of Research on the Structure of Matter (FJIRSM) of Chinese Academy of Sciences reported a lantern-shaped calix[4]resorcinarene-based porous organic cage (POC, namely, CPOC-101) can exhibit eight distinct solid-state solvatomorphs via crystallization in different solvents. This POC solvatomorphism has a significant influence on their gas sorption capacities as well as separation abilities.
The researchers found that the apparent Brunauer-Emmett-Teller (BET) surface area determined by nitrogen gas sorption at 77 K for CPOC-101α crystallized from toluene/chloroform is up to 406 m2 g-1, which is much larger than the rest of CPOC-101 solvatomorphs with BET values less than 40 m2 g-1.
More interestingly, C2H2 and CO2 adsorbed capacities, in addition to the C2H2/CO2 separation ability at room temperature for CPOC-101α, are superior to those of CPOC-101β crystalized from nitrobenzene, the representative of POC solvatomorphs with low BET surface areas.
To understand the mechanism of the higher affinity toward C2H2 over CO2 within CPOC-101, the researchers computed the interaction energies between the optimized cage host and gas guests by the first-principles dispersion-corrected density functional theory (DFT-D) calculations.
The hydrogen-bond number (6 for C2H2 and 5 for CO2), the average hydrogen-bond length (3.12 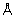 for C2H2 and 3.26
for C2H2 and 3.26 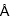 for CO2), and the calculated interaction enthalpies highly indicated that the host-guest interaction between the C2H2 molecule and CPOC-101 is much stronger than that of CO2.
for CO2), and the calculated interaction enthalpies highly indicated that the host-guest interaction between the C2H2 molecule and CPOC-101 is much stronger than that of CO2.
This study reveals the possibility of adjusting gas sorption and separation properties of POC materials by controlling their solvatomorphs.
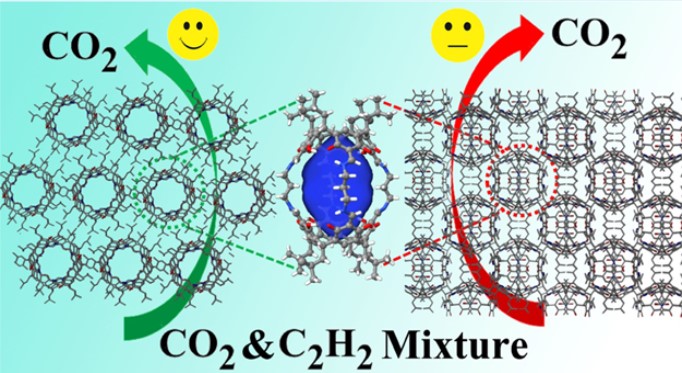
CPOC-101 for C2H2/CO2 separation. (Image by Prof. YUAN's Group)
Contact:
Prof. YUAN Daqiang
Fujian Institute of Research on the Structure of Matter
Chinese Academy of Sciences
Email: ydq@fjirsm.ac.cn