Cesium (137Cs)possesses long half-life and emits high-energy γ rays. Moreover, it is highly soluble and easy to migrate in the environment and biosphere, posing serious threats to human health and environmental safety. 137Cs is one of the main sources of radioactivity in spent fuel. It is very important to remove the strongly radioactive 137Cs+ from high-level liquid wastes (HLLWs) to reduce its radioactivity level before any further disposal. HLLWs are generally acidic.
However, the effective capture of Cs+ ions from acidic solutions remains a very challenging task. Most current materials don’t well perform under the acidic conditions due to the stability issue of materials and the strong competition of protons. On the other hand, the Cs+ capture mechanism under strongly acidic conditions is still unclear.
In a study published in Nat. Commun., Prof. FENG Meiling and her colleagues from Prof. HUANG Xiaoying’s research group at Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, reported a robust layered compound KInSnS4 (InSnS-1) that can effectively capture Cs+ under both neutral and acidic conditions.
The researchers found that InSnS-1 has excellent acid and irradiation resistances, maintaining its layered network under strongly acidic (1 - 4 mol/L HNO3) and irradiation (200 kGy γ irradiation) conditions. InSnS-1 exhibits fast adsorption kinetics, high adsorption capacities forCs+ ions under both neutral and acidic (1 mol/L HNO3) conditions, and easy desorption and reuse. It can realize effective separation of Cs+/Mn+ (Mn+ = Na+, Sr2+, Ca2+and La3+) under strongly acidic conditions (1 and 3 mol/L HNO3).
They also unveiled that InSnS-1 can also be used as a stationary phase in ion exchange column for the convenient and efficient treatment of neutral or acidic wastewater containing high concentrations of Cs+ ions.
Meanwhile, the researchers directly visualized the entry of Cs+ and H3O+ ions by single-crystal structural analysis, and illuminated the underlying mechanism of selective Cs+ capture from acidic solutions at the molecular level.All promising features of InSnS-1 for the Cs+ capture originate from the extremely strong interactions between soft S2- of sulfide layers and relatively soft Cs+, the adjustable interlayer spacing and structural flexibility of InSnS-1 under acidic conditions.
Besides, the researchers revealed the non-negligible facilitating role of H3O+ in the selective capture of Cs+ ions under acidic conditions, and the important influence of the shortest K-S distance of K+-directed metal sulfides on the ion exchange performance.
This study represents a breakthrough in the research of the Cs+ capture under highly acidic conditions through developing an effective material and providing a deep insight into the mechanism of selective Cs+ capture from the view of microstructural illumination.It is pioneering to systematically study the selective capture of Cs+ by metal sulfides under strong acidic conditions, which will shed light on the design of novel acid-tolerant sulfide-based ion exchange materials for radiocesium decontamination with practical applications.
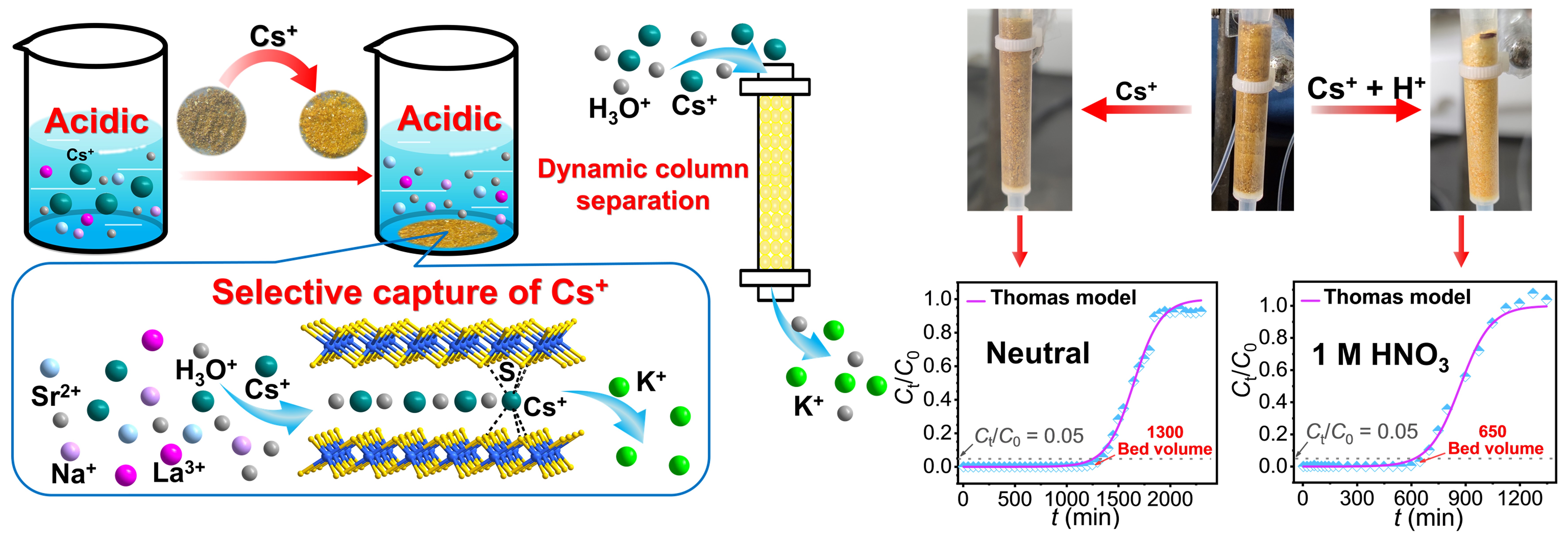
Illustration of the Cs+ capture study under acidic conditions (left) and ion exchange column experiment (right) (Image by Prof. FENG)
Contact:
Prof. FENG Meiling
Fujian Institute of Research on the Structure of Matter
Chinese Academy of Sciences
Email: fml@fjirsm.ac.cn