Ion-interference therapy which utilizes ions to disturb intracellular biological processes provides inspiration for tumor therapy. Artificially reversing osmotic pressure by transporting large amounts of physiological ions to tumor cells is a straightforward yet low-toxic strategy for ion-interference therapy.
However, the short circulation lifetime, dose-limiting systemic toxicity, and poor selectivity toward tumors make it unavailable to achieve the single ion delivery.
In a study published in ACSNano, the research group led by Prof. LIU Xiaolong from Fujian Institute of Research on the Structure of Matter of the Chinese Academy of Sciences & Mengchao Hepatobiliary Hospital of Fujian Medical University developed a tetrasulfide(-s-s-s-s-)-bridged virus-mimicking hollow mesoporous silica (ssss-VHMS) moiety to skillfully delivered NaCl nanocrystals to tumor sites and sequentially realized the explosive release of Na+ inside tumor cells.
Owing to the spike surface-assisted endocytosis, the ssss-VHMS-wrapped NaCl nanocrystals could rapidly invade tumor cells by passing Na+/K+-ATPase transmembrane ion transporters.
Besides, the intracellular overproduced glutathione (GSH) of tumor cells could trigger the rapid degradation of ssss-VHMS, which would not only remarkably deplete the GSH but also explosively release the Na+, resulting in the osmolarity surge accompanied by the reactive oxygen species (ROS) generation.
Specifically, the researchers demonstrated that the cell swelling, ROS storm, and GSH exhaustion induced by NaCl@ssss-VHMS efficiently eradicated tumor cells, due to the caspase-1-dependent pyroptosis, caspase-3-dependent apoptosis, and glutathione peroxidase 4 (GPX4)-dependent ferroptosis, respectively, thus synergistically inhibiting tumor growth.
This discovery provides a perspective for exploring synergistic ion-interference therapy.
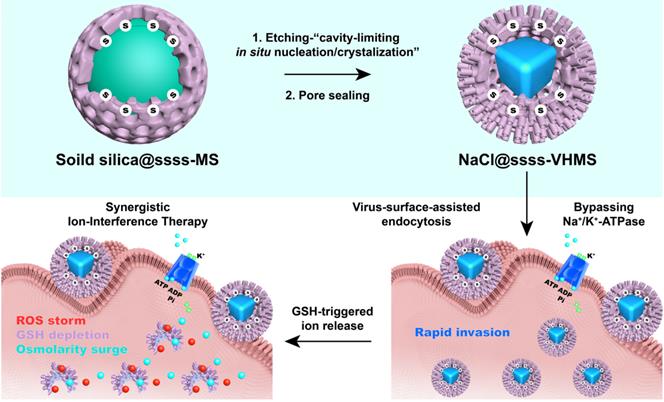
Schematic Illustration of the Research (Image by Prof. LIU’s Group)
Contact:
Prof. LIU Xiaolong
Fujian Institute of Research on the Structure of Matter
Chinese Academy of Sciences
Email: liuxl@fjirsm.ac.cn