The electrochemical CO2 reduction reaction (CO2RR) plays an important role in addressing climate-change issues and global energy demands as part of a carbon-neutral energy cycle. Selective conversion of CO2 to multi-carbon products remains a challenge due to the sluggish kinetics during C-C coupling.
Cu-based materials, including metallic Cu, Cu oxides, and Cu-containing molecules, have been proved to be capable of producing C2/C2+ hydrocarbons in considerable amounts. However, these conventional Cu-based electrocatalysts usually suffer from the insufficient clarity of the active sites and low selectivity. Copper-based tandem catalysts with well-defined Cu coordination environment for CO2RR are highly desirable, due to their unique geometric-electronic properties and helpfulness for revealing structure-property correlation.
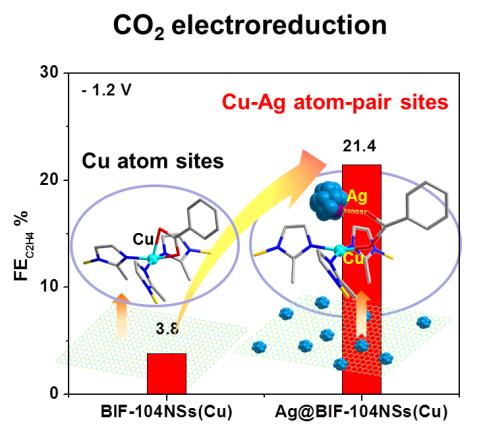
In a study published in Advanced Energy Materials, the research group led by Prof. ZHANG Jian from Fujian Institute of Research on the Structure of Matter of the Chinese Academy of Sciences constructed a tandem catalyst (Ag@BIF-104NSs(Cu)) at atomic configuration scale to enhance the selectivity and activity for CO2RR to C2H4, providing fundamental comprehension for the C-C coupling in the CO2 reduction process.
The researchers constructed the composite material (Ag@BIF-104NSs(Cu)) as a tandem catalyst, where the support of BIF-104(Cu) nanosheet provides the well-defined environment of Cu atoms. Ag nanoparticles were attached to Cu atoms via coordination effect with the abundant carboxylic functional ligands in the BIF-104NSs(Cu) surface.
By electrochemistry performance test, the researchers found that this tandem catalyst Ag@BIF-104NSs(Cu) could efficiently enhance the faradic efficiency of C2H4 products (FE of 21.43 %), which is almost 6-folds enhancements of BIF-104NSs(Cu) alone (3.82 %).
Through electrochemistry mechanism analysis, they discovered that Ag@BIF-104NSs(Cu) tandem catalysts provide more effective active sites and faster electron transfer in electroreduction CO2 process, leading to enhanced CO2RR activity and C2H4 selectivity.
Besides, density functional theory calculations revealed that the Ag sites in the Ag@BIF-104NSs(Cu) catalysts can efficiently reduce CO2 and enrich surface coverage of *CO, which subsequently migrated to the atomic proximity Cu sites. Therefore, the Cu-Ag atom pair is responsible for the C-C coupling of the local enriched *CO and further production of C2H4.
This study provides a new approach for developing high-performance electrochemical CO2 reduction tandem catalysts to prepare C2 products.
Schematic Illustration of the Research(Image by Prof. ZHANG’s group)
Contact:
Prof. ZHANG Jian
Fujian Institute of Research on the Structure of Matter
Chinese Academy of Sciences
Email: zhj@fjirsm.ac.cn
.
