Lithium bis(trifluoromethanesulfonyl)imide, commonly known as LiTFSI, and its analogs are critical high-end electrolytes for lithium batteries and solar cells. However, the current commercialization of LiTFSI through thermal chemical synthesis relies on the use of NH3 intermediates, involving multiple catalytic and purification processes, leading to substantial carbon emissions. Therefore, developing a method for the direct synthesis of LiTFSI from N2 under mild conditions becomes particularly important.
In a study published in Nature Catalysis, Prof. WANG Yaobing and his team from Fujian Institute of Research on the Structure of Matter of the Chinese Academy of Sciences, proposed a cascade electrochemical synthesis strategy based on Li-N2 batteries, and achieved efficient electrochemical synthesis of various nitrogen-containing compounds, including LiTFSI.
The specific strategy includes catalytically reducing N2 to Li3N during discharge, acylating Li3N to form LiTFSI and the byproduct LiCl, and oxidizing LiCl during charging to complete the synthetic cycle.
The researchers demonstrated the electrocatalytic reduction of N2 to Li3N through X-ray diffraction, low-temperature transmission electron microscopy combined with EDS spectra. They confirmed the feasibility of the S-N acylation reaction between Li3N and CF3SO2Cl through nuclear magnetic resonance, mass spectrometry, and Fourier-transform infrared spectroscopy. Based on the color change of methyl orange from red to colorless during the charging process, the researchers proved that the byproduct LiCl was oxidized to Cl2.
The experimental results indicated that, under optimized conditions, the catalytic reduction efficiency from N2 to Li3N reached 53.2%, the conversion efficiency from N2 to LiTFSI was 48.9%, and the energy efficiency of electrochemical synthesis of LiTFSI reached 3.0%.
Moreover, the researchers utilized a flow cell device to achieve continuous electrochemical synthesis of LiTFSI, demonstrating the practical significance of this strategy in production.
By expanding the substrate scope, the researchers provided a pathway for the direct electrochemical synthesis of analogs with different N-X bonds (X = S, C, etc.) and metal cations (Li+, Zn2+, etc.), proving the scalability of the strategy.
This study presents a comprehensive electrochemical synthesis scheme for the practical production of nitrogen-containing chemicals, which offers a promising approach to synthesize high-end electrolytes with enhanced nitrogen atom efficiency.
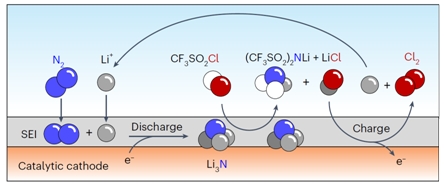
Schematic representation illustrating the cascade LiTFSI synthesis in a Li-N2 battery (Image by Prof. WANG Yaobing’s group)
Contact:
Prof. WANG Yaobing
Fujian Institute of Research on the Structure of Matter
Chinese Academy of Sciences
Email: wangyb@fjirsm.ac.cn