The sustainable development of the nuclear industry is closely linked to the proper disposal of radioactive waste. 137Cs, one of the major sources of gamma radioactivity in spent fuel, can greatly harm the ecosystem once it is released into the environment due to its strong radioactivity, high solubility and easy migratory. Highly selective capture of 137Cs+ ions is urgently needed for remediation of environmental radioactive contamination and spent fuel treatment.
However, complex environments pose significant challenges for the selective separation of radiocesium from radioactive liquid wastes. Due to strong Coulombic interactions, the adsorption performance of conventional Cs+ adsorbents is usually greatly affected by competing ions, especially high-valency metal ions.
In a study published in Nat. Commun., Prof. FENG Meiling et al. from Prof. HUANG Xiaoying’s research group at Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, proposed a strategy for the construction of “inorganic ion-imprinted adsorbents” with superior capabilities of ion recognition-separation.
This strategy is expected not only to reduce the constraints imposed by the type of functional groups or functional monomers on the development of imprinted polymers, but also to combine the high selectivity advantage of imprinted adsorbents with the excellent irradiation resistance, environmental compatibility, and adsorption efficiency of inorganic adsorbents.
Researchers found that the new “inorganic ion-imprinted adsorbents” are more suitable for radioactive waste treatment. Based on this strategy, the metal sulfide Cs2.33Ga2.33Sn1.67S8·H2O (FJSM-CGTS) with an “imprinting effect” on Cs+ had been prepared. The K+-activation product of FJSM-CGTS, Cs0.51K1.82Ga2.33Sn1.67S8·H2O (FJMS-KCGTS), has a maximum adsorption capacity of 246.65 mg/g for Cs+. The selectivity of Cs+ ions is greatly improved by the synergistic effect of the “imprinting effect” and the affinity of soft basic S2- sites for Cs+ ions.
Researchers revealed that FJSM-KCGTS can capture Cs+ ions highly selectively in the presence of high concentrations of competing ions (K+, Ca2+, Na+, Mg2+, Sr2+, and Eu3+). It exhibits excellent treatment capabilities for actual 137Cs waste solutions, which can reduce 137Cs activity of waste solutions by more than 98%. Futhermore, FJMS-KCGTS can be used as a stationary phase for ion exchange columns to dynamically treat Cs+-containing waste solutions quickly and easily.
Importantly, researchers obtained the single-crystal structure of the inorganic ion-imprinted adsorbent and its K+-activation and Cs+-capture products, and the coordination modes for Cs-S have been clarified, which reveals the “ion-imprinting” process of the inorganic ion-imprinted adsorbent for the capture of Cs+ in an intuitive and clear way.
The excellent selectivity for Cs+ originates from the strong interaction of the anionic sulfide layer with Cs+ which has been confirmed by density functional theory (DFT) calculations. The selective adsorption mechanism and structure-function relationship are clarified by structural comparative analysis and theoretical calculations from perspectives of structure and energy.
The relatively robust wave layered structure and the strong interaction of the soft basic S2- sites with Cs+ ions give the anionic layer of FJSM-CGTS a spatial confinement effect on Cs+ ions. This enables the material to exhibit the “imprinting effect” and selective trapping ability for Cs+ ions, thus confirming the effectiveness of the synthetic strategy of constructing “inorganic ion-imprinted adsorbents”.
This study not only contributes significantly to the scientific understanding of the structure-function relationship, but also opens up new possibilities for environmental radioactive contamination remediation and spent fuel disposal.
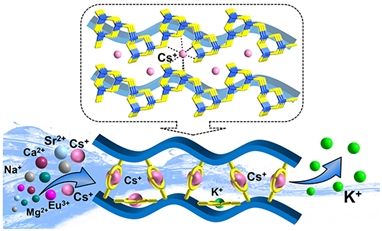
Illustration of the Cs+ capture by “inorganic ion-imprinted adsorbent” in the presence of high concentrations of competing ions (Image by Prof. FENG).
Contact:
Prof. FENG Meiling
Fujian Institute of Research on the Structure of Matter
Chinese Academy of Sciences
Email: fml@fjirsm.ac.cn