Volatile organic compounds (VOCs) are precursors of O3 and particle matters, causing a serious threat to human health and ecological environment. Catalytic oxidation is one of the most efficient technologies to control and eliminate VOCs, which converts VOCs to CO2 and water in a relatively mild condition. The key issue of this technology is highly active and stable catalysts.
CeO2 is a good candidate to be used in VOCs degradation due to the excellent oxygen storage and release capacity and favorable redox behavior. However, pure CeO2 catalysts generally have low activity in VOCs degradation. Researchers devote themselves in the modification methods such as element doping and noble metal loading, which may increase costs. Therefore, it is still worth to explore new synthesis approach to obtain CeO2 catalysts for VOCs degradation and reveal the reason for high activity.
In a study published in SEPARATION AND PURIFICATION TECHNOLOGY, the research group led by Prof. LU Canzhong from Fujian Institute of Research on the Structure of Matter of the Chinese Academy of Sciences reported a highly active CeO2 nanowire catalyst prepared by the freeze-drying method for toluene catalytic oxidation.
The porous structure of the CeO2 nanowire kept during the freeze-drying process is more conducive to fill toluene molecules for full contact between catalysts surfaces and toluene, Moreover, its large number of oxygen vacancies, the big pore volume, the more surface active oxygen and the lower temperature reducibility lead to its high activity for toluene oxidation. It shows catalytic oxidation of toluene with T90 of 220 ◦C, and the corresponding mineralization at 227 ◦C (WHSV = 6000 mL g-1h−1), which is much better than that of commercial CeO2 or CeO2 prepared by MOFs template, precipitation and hydrothermal methods.
Specially, CeO2 nanowire catalyst has good catalytic stability and excellent water resistance. With 5 Vol. % water vapor addition at 231 ◦C, the toluene conversion may decrease from to 90% to 70%, and its conversion may recover to 90% in an hour after turning off the water vapor.
Besides, the in situ Diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) analysis indicated that the toluene catalytic oxidation reaction may undergo the steps as toluene → benzaldehyde → benzoate species → maleic acid → CO2 and H2O.
This study provides an approach to synthesize highly active CeO2 catalysts with new structures for toluene oxidation, and opens an efficient way for the construction of CeO2 based composite catalysts for VOCs degradation
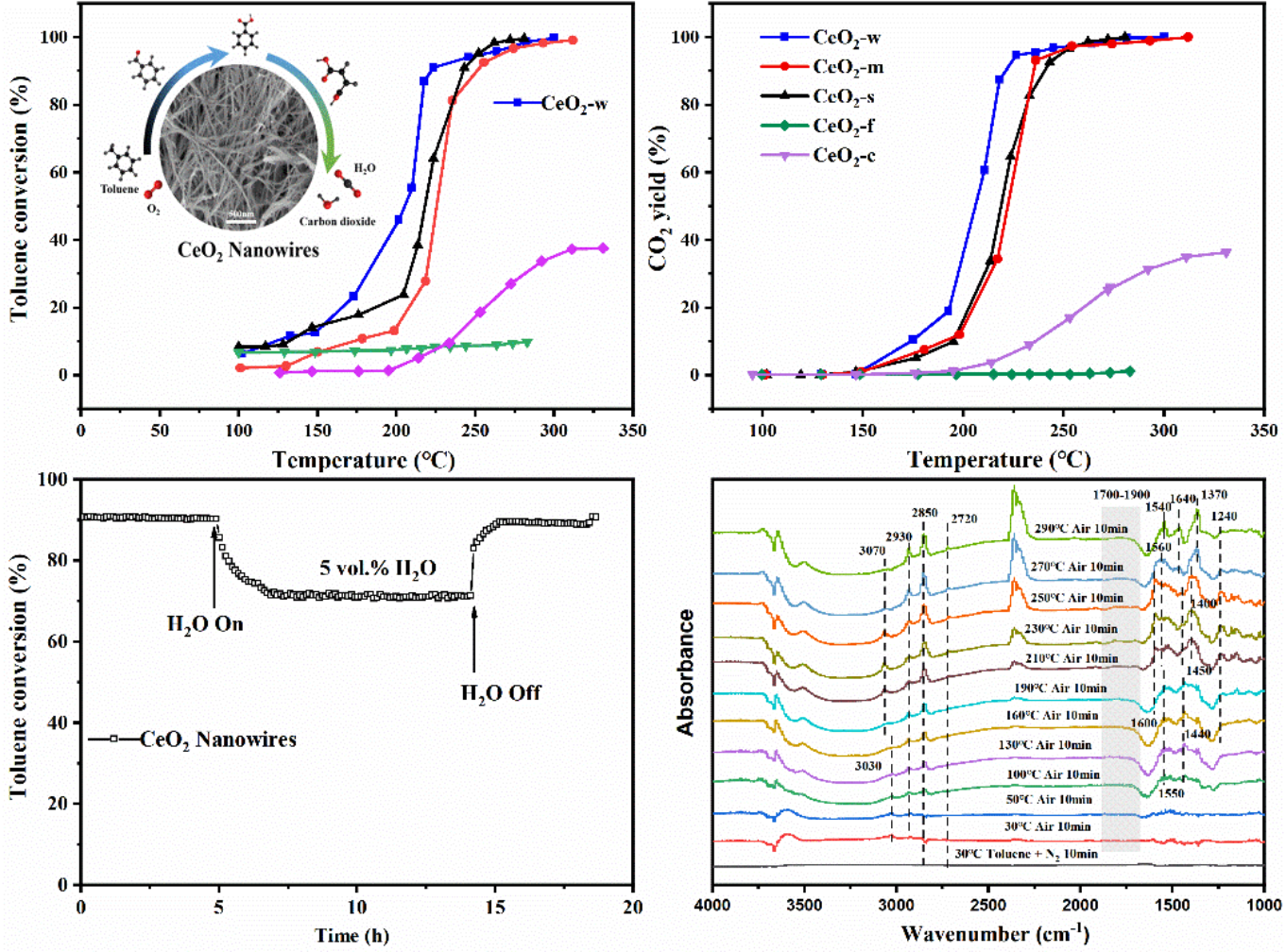
Schematic of the toluene catalytic oxidation performance of CeO2 nanowire catalysts: the conversion and CO2 yield, the effect of water vapor, and the In situ DRIFTS analysis. (Image by Prof. LU’s Group)
Contact:
Prof. LU Canzhong
Fujian Institute of Research on the Structure of Matter
Chinese Academy of Sciences
Email: czlu@fjirsm.ac.cn