The design and synthesis of hybrid borates by the organic ligand modification method are urgent and undeveloped areas of research. It is difficult to directly integrate organoboronic acids within inorganic borate chemistry by adopting the traditional preparation approaches.
In a study published in Angew. Chem. Int. Ed., the research group led by Prof. ZHANG Jian from Fujian Institute of Research on the Structure of Matter of the Chinese Academy of Sciences, reported a facile synthetic method to synthesize a large family of pyrazole molecule-protected boratesin a rapid and precise manner under mild conditions.
The researchers identified the unique cyclic eight-membered B4O4-ring as the cluster core for all these hybrid borates with two different conformations (boat and crown).This strategy can be applied to a system of pyrazolyl molecules to generate such hybrid borates in two independent routes from organoboronic or inorganic boric acids.
The researchers proposed the mechanism of ‘click reaction’ between boric acid and pyrazole induced by copper ions based on the synthetic conditions and the structure of intermediate. Due to the bimetallic Cu sites and the functional surfaces, these materials can be used as electrocatalysts for CO2 reduction reaction and efficiently enhance the selectivity of HCOOH and C2H4.
In recent decades, the continuous innovation of synthesis approaches of borate has promoted the development of solid-state chemistry and materials science. The introduction of templates/structure-directing agents, such as organic amines, inorganic cation, and metal complexes, has greatly enriched the types of borates. However, due to the absence of rational mechanism guidance, synthetic borate crystals seem to be approaching the limits of their structural diversity.
Inspired by the concept of click chemistry, the researchers found that copper ions could induced ‘click reaction’ between boronic acid and pyrazole to synthesis organic molecule-protected borates precisely under mild conditions with high yield.
On this basis, the researchers showed how these compounds can be precisely generated by two independent routes as well as how this strategy can be applied to a system of pyrazolyl molecules. Based on the synthetic condition and the structure of intermediate, they find that the formation of these unique structures is driven by Lewis acid-base interaction and coordination interactions, in which copper ions paly an important role in promoting the reaction. Their strategy can be regarded as a typical template technique for pyrazolyl molecule-protected borates.
Furthermore, due to the bimetallic Cu sites and the functional surfaces, the researchers systematically investigated their electrocatalytic performances for CO2 reduction reaction. MePzBor-3I could efficiently catalyze CO2 to produce HCOOH and C2H4 with the faradic efficiency (FE) of 55.72% and 23.90%, respectively.
Based on the synthetic condition and the structure of intermediate, Lewis acid-base interaction and coordination interactions are the driven forces for this ‘click reaction’ between boric acid and pyrazole.
This study opens a new route toward the construction of novel organic-inorganic hybrid borates.
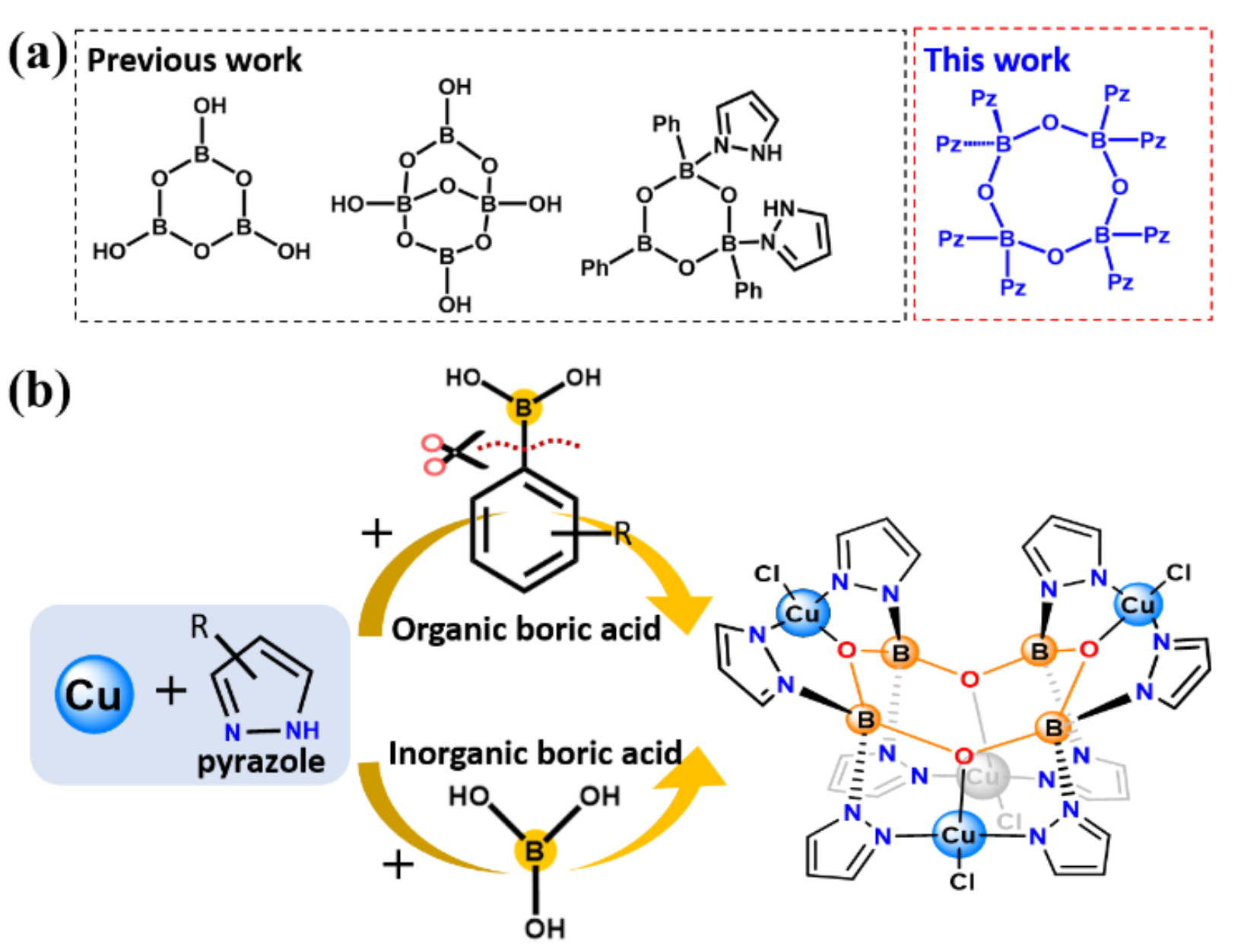
Schematic illustration of (a) the comparation of geometric configuration between B3O3 rings and B4O4 ring; (b) Schematic for Cu ions induced ‘click reaction’ between boric acid and pyrazole.(Image by Prof. ZHANG’s group)
Contact:
Prof. ZHANG Jian
Fujian Institute of Research on the Structure of Matter
Chinese Academy of Sciences
E-mail: zhj@fjirsm.ac.cn