Constructing biomimetic local microenvironments is one of the important strategies to improve the electrocatalytic performances, such as in electrochemical CO2 reduction (ECR). However, effectively customizing these microenvironments remains a significant challenge.
In a study published in Journal of Colloid And Interface Science, a research group led by Prof. ZHU Qilong from Fujian Institute of Research on the Structure of Matter of the Chinese Academy of Sciences disclosed the promotion effect of the local hydroxyl microenvironment in CO2-to-CO electroconversion through tailoring three single-molecular heterostructures with Co-N2O2 configurations.
The researchers synthesized three planar conjugated model molecules, namely, Co-salophen, Co-salophen-OH3 with ortho-hydroxyl groups and Co-salophen-OH4 containing meta-hydroxyl groups, as confirmed by the high-resolution mass spectra and crystallographic analysis. Subsequently, the CNT-heterostructured electrocatalysts were nondestructively constructed as a customized organic–inorganic hybrid platform to decode their structure–activity relationship in ECR.
Concretely, compared with the maximum Faradaic efficiency (FE) of 62% for CO presented by Co-salophen/CNT, the designed Co-salophen-OH3/CNT, featuring ortho-hydroxyl groups at the Co-N2O2 structural opening, shows remarkable CO2-to-CO electroreduction activity across a wide potential window, with the FE of CO up to 95%.
Besides, the researchers found that the closer the hydroxyl groups are to the Co-N2O2 active sites, the more pronounced improvements in CO partial current density and FE are realized.
The mechanism study of deuterium kinetic isotope experiments and theoretical calculations unveiled that the adjoining hydroxyl groups greatly optimize the reaction path of the ECR through their unique proton ferry capacity.
This finding demonstrates a promising molecular design strategy for enhancing electrocatalysis.
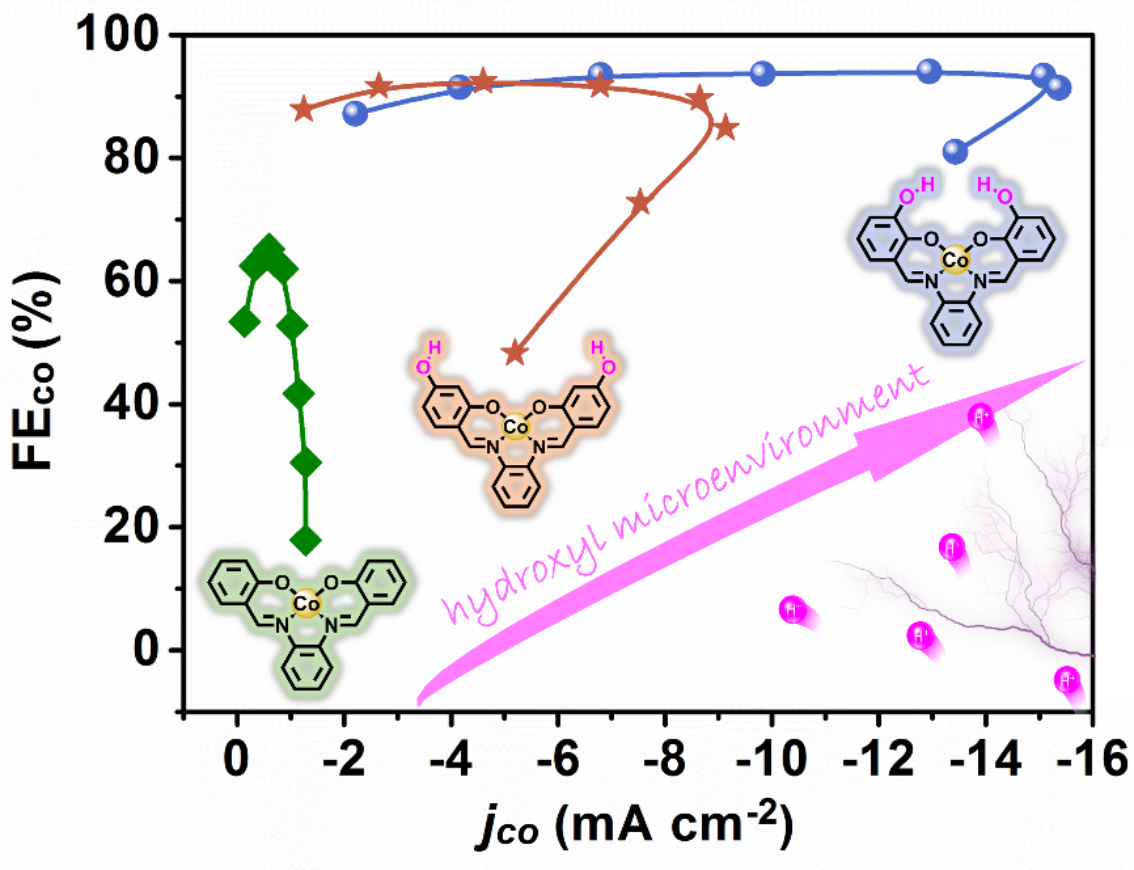
Illustration of the Research(Image by Prof. ZHU’s group)
Contact:
Prof. ZHU Qilong
Fujian Institute of Research on the Structure of Matter
Chinese Academy of Sciences
Email: qlzhu@fjirsm.ac.cn