Zeolites are extensively utilized crystalline microporous materials in the chemical and petrochemical industries. Among these, zeolites containing multivalent transition metal ions have gained significant interest due to their distinctive chemical and physical properties. In particular, small-pore Cu-CHA zeolites have demonstrated remarkable catalytic efficiency in industrial DeNOx processes and the partial oxidation of methane to methanol.
The activation of these Cu-based zeolite catalysts involves an autoreduction or self-reduction reaction, where a portion of Cu(II) species is spontaneously reduced to Cu(I) species at elevated temperatures under vacuum or inert gas conditions. Despite the extensive studies, a comprehensive computational investigation into the reaction pathways of autoreduction and the intricate molecular-level mechanisms governing this process remains elusive.
In a study published in Chemistry – An Asian Journal, the research team led by Associate Prof. LIU Chong and Prof. ZHUANG Wei from the Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, conducted a computational investigation into the detailed mechanism of copper autoreduction in Cu-CHA zeolites.
The researchers systematically explored two primary reduction mechanisms. In Mechanism I, two [CuOH]+ species undergo dehydration to form [Cu-O-Cu]2+, and subsequently the reaction of two [Cu-O-Cu]2+ species produces molecular O2. In Mechanism II, the production of O2 occurs via [CuO]+ intermediates, with the generation of [CuO]+ being the critical step.
The researchers highlighted the importance of the O–O distance in reactive intermediates, which is influenced by the spatial positioning of lattice aluminum sites hosting the original Cu(II) species.
The researchers also found that an optimal O–O distance significantly reduced the energy barrier for O2 production, emphasizing the structural role of the zeolite framework in facilitating the autoreduction process.
Besides, the researchers provided a detailed molecular-level understanding of the copper autoreduction mechanism in Cu-CHA zeolites, illustrating how the structural configuration of the zeolite framework affects catalytic activity.
These findings deliver actionable insights for the rational design and optimization of Cu-based zeolite catalysts, paving the way for enhanced performance in industrial catalytic applications.
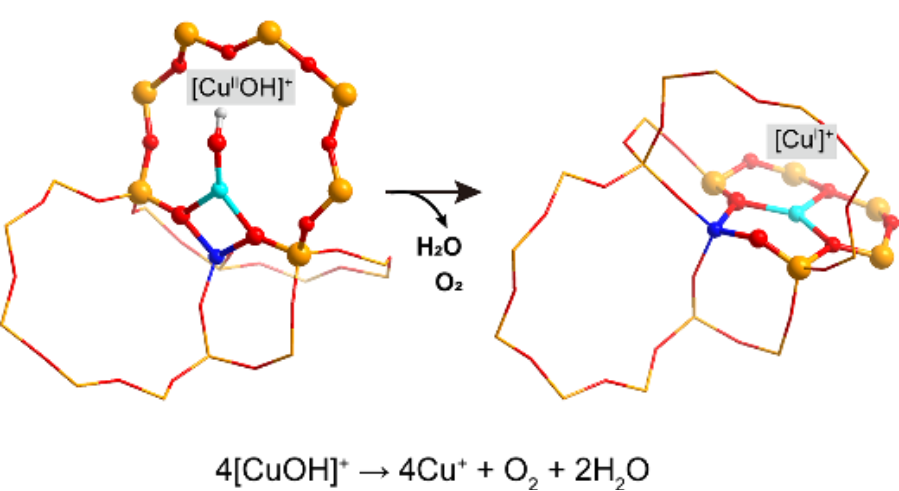
Autoreduction reaction in Cu-CHA zeolites(Image by Prof. ZHUANG’s group)
Contact:
Prof. ZHUANG Wei
Fujian Institute of Research on the Structure of Matter
Chinese Academy of Sciences
Email:wzhuang@fjirsm.ac.cn