As ultra-small nanoparticles with uniform sizes and well-defined structures, ligand-protected coinage metal nanoclusters show distinctive electronic structures and physicochemical properties in contrast to those of bulk nanomaterials due to quantum size effects. Nevertheless, most of them suffer from the issues such as facile dissociation in solutions and poor thermodynamic stability, which greatly restrict their practical applications as optoelectronic materials.
Peripheral protection ligands are the key elements in coinage metal clusters, thus exerting a crucial impact on the chemical properties and stability. In most cases, the protective effect of ligands with simply bridging character could not endow sufficient robustness and internal stability of nanoclusters. To provide adequate shielding and protecting effects for metal cluster kernels, bifunctional ligands having both bridging and chelating characteristics are better candidates for constructing robust metal nanoclusters with highly efficient luminescence. Chelating and bridging effect of bifunctional ligands not only enhances greatly the surface rigidity of metal nanoclusters beneficial for stabilizing the internal structures, but also minimizes non-radiative thermal relaxation processes on nanocluster surfaces, substantially improving intrinsic luminescence efficiency of nanoclusters.
In a study published in Science Advances, Prof. CHEN Zhongning from Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences and Prof. SUN Di from Shandong University elaborately designed a stable bifunctional diphosphine (2-Ph2PC6H4PH2) featuring primary and tertiary phosphorus donors as a unique chelator, which shows synergistic bridging and chelating characteristics for creating ultrastable silver nanoclusters with metallic aromaticity.
Upon deprotonation, both -P2- and -PPh2 donors simultaneously bridge and chelate multiple metal ions to give m5-P2- capped M5 pentagons through strong Ag-Ag interactions, thus resulting in arc surfaces or spherical caps, which serve as building blocks for the construction of spherical architectures with specific polyhedral nanoclusters.
By precisely controlling the deprotonation rate of 2-Ph2PC6H4PH2 and introducing organic or inorganic templates, the researchers synthesized and characterized two novel silver nanoclusters (Ag30 and Ag32) possessing fascinating icosidodecahedral Ag30 shells with an Ih symmetry. The distinctive coordination geometry of 2-Ph2PαC6H4Pβ2- in m5-η1(Pβ),η2(Pα,Pβ) coordination mode endows Ag30 and Ag32 clusters with spherical metallic aromaticity, which plays a pivotal role in the exceptional stability of the two clusters. As each 2-Ph2PαC6H4Pβ2- ligand possesses two negative charges and bonds to five silver(I) atoms via the -Pβ2- donor to provide three lone pairs of electrons to form a 6c-6e- delocalized bond, twelve such 2-Ph2PC6H4P2- ligands on the Ag30 shell of Ag30 or Ag32 afford 72 bonding electrons (2×(5+1)2), which is consistent with the 2(N+1)2 rule of spherical aromaticity.
Ag30 exhibits high-efficiency NIR electroluminescence in solution-processed organic light-emitting diodes (OLEDs) with peak external quantum efficiency (EQE) of 15.1%, representing a significant progress in the application of silver nanoclusters to NIR-emitting devices.
This study not only broadens the scope of protective ligands available for coinage metal clusters to get profound insights into synthesizing coinage metal clusters endowed with metal aromaticity and excellent stability, but also provides crucial guides for the practical application of functional nanoclusters in optoelectronic devices.
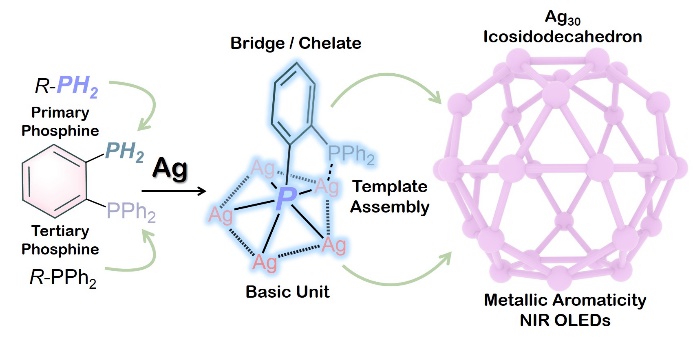
Icosidodecahedral silver nanoclusters demonstrate metallic aromaticity and exhibit near-infrared luminescence properties with OLEDs application. (Image by Prof. CHEN’s group)
Contact:
Prof. CHEN Zhongning
Fujian Institute of Research on the Structure of Matter
Chinese Academy of Sciences
Email: czn@fjirsm.ac.cn