In principle, the reaction of 1,1,2,2-tetrasubstituted donor-acceptor cyclopropanes ( DACs ) produces tetrasubstituted stereocenters, which is of great significance for the synthesis of related complex functional molecules. However, despite the great achievements, in this field, the highly efficient catalytic asymmetric transformation of 1,1,2,2-tetrasubstituted DACs remains anunsolved challenge, although many reports using tetrasubstituted DACs that afford racemic compounds have beenreleased. The challenges might arise from the additional steric bulkiness and the increased sluggishness for ring opening when cyclopropanes having two geminal donor groups are used.
In a study published in Journal of the American Chemical Society, Prof. FANG Xinqiang 's group of Fujian Institute of Material Structure, Chinese Academy of Sciences realized copper-catalyzed asymmetric nucleophilic ring-opening reaction of tetrasubstituted cyclopropanes to prepare α-tertiary amines. A series of novel reactions have been realized by using in-situ generated copper allene methylene intermediates.
Researchers completed the efficient nucleophilic ring-opening reaction of tetrasubstituted donor-acceptor cyclopropanes containing terminal alkyne units by using this strategy, and generated a variety of α-tertiary amines with high enantioselectivity, which is an effective supplement to traditional Lewis acid catalysis.
The key intermediates are copper allenylideneintermediate I and its resonance structure of 1,3-zwitterion II,and such a reaction pattern represents a different approach torealizing asymmetric reactions of DACs. This mode provides a new method for the asymmetric reaction of donor-acceptor cyclopropane.The corresponding products contain amine, alkyne and ester functional groups, which can be further transformed, such as the asymmetric synthesis of nitrogen-containing bioactive compounds and drug molecules.
Mechanistic studies indicated that the zwitterionic intermediate bearing a copper-acetylide unit plays a key role in the process, which represents a new mode for achieving catalytic asymmetric transformation of DACs.
This study reveals the copper-catalyzed asymmetric aminative ring opening of tetrasubstituted alkynyl DACs that delivers a myriad of α-tertiary amines with high levels of enantioselectivities. The alkyne, amine, and ester moieties within the products enable diverse further applications, including the asymmetric synthesis of bioactive molecules.
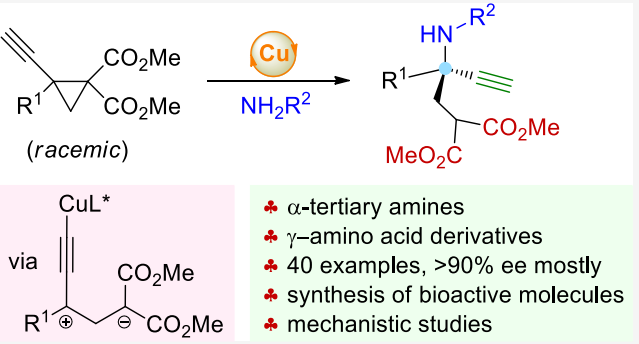
Asymmetric nucleophilic ring opening of tetrasubstituted cyclopropanes (Image by Prof. FANG Xinqiang's group)
Contact:
Prof. FANG Xinqiang
Fujian Institute of Research on the Structure of Matter
Chinese Academy of Sciences
Email:xqfang@fjirsm.ac.cn