Recent advancements in the domain of renewable energy technologies have precipitated an augmented demand for efficient and economical energy storage solutions. Aqueous zinc-ion batteries (AZIBs) are esteemed for their safety, economy, and environmental benefits. Nevertheless, the reversibility and stability of AZIBs are considerably constrained.
The advent of organic aqueous electrolytes has signified a substantial advancement in battery technology over the past decade. These additives have proven to be remarkably efficacious in modulating the solvation structure and establishing interfacial layers. The efficacy of AZIBs can be optimized through the enhancement of safety, electrochemical reversibility, and ion mobility. Nevertheless, a delicate equilibrium must be maintained between performance and operational stability, as certain organic additives may precipitate undesirable side reactions or intricate phase behavior, thereby nullifying their advantageous effects.
In a study published in Journal of Colloid and Interface Science, the research group led by Prof. ZHANG Yining from Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, has reported a novel approach to enhancing the performance of AZIBs through the introduction of triethyl 2-phosphonopropionate (Tp), a high-dipole-moment electrolyte additive. The study's findings indicate that Tp effectively replaces free water in the electrolyte, thereby suppressing hydrogen evolution and zinc corrosion while promoting reversible zinc deposition and mitigating dendrite growth.
In order to ascertain the optimal concentration ratio, the researchers prepared Tp electrolyte solutions with varying volume ratios. In order to combat the free water-induced side reactions (e.g., hydrogen precipitation and zinc corrosion) and the disordered growth of zinc dendrites, Tp effectively replaces the free water in the electrolyte through strong ion-dipole interactions to alter the solvated structure. The high binding energy between Tp and zinc foil ensures that Tp is firmly attached to the zinc anode surface and inhibits dendrite growth. Theoretical and experimental results show that the addition of Tp significantly changes the hydrogen bonding network structure in the electrolyte.
Furthermore, the researchers compared the performance differences between Tp electrolytes with different volume ratios and the initial electrolyte in practical electrochemical tests. The Zn//Na2V6O16 with the optimal concentration of Tp electrolyte exhibited 92% capacity retention after 4,000 cycles at a current density of 3 A g-1. In contrast, Zn//Na2V6O16 with the initial electrolyte exhibited only 70% capacity retention after 600 cycles and a significant decrease in Coulombic efficiency. These findings suggest that the electrolyte incorporating Tp has superior cycling stability and multiplicity performance compared to the initial electrolyte.
Furthermore, the researchers appraised the practical application potential of the Tp electrolyte. A Zn//Na2V6O16 flexible pack battery with Tp electrolyte was prepared and successfully illuminated an LED light, thereby demonstrating the potential value of the Tp electrolyte in production applications.
This study emphasizes the importance of high dipole moments in additive strategies that contribute to the practical application of AZIBs.
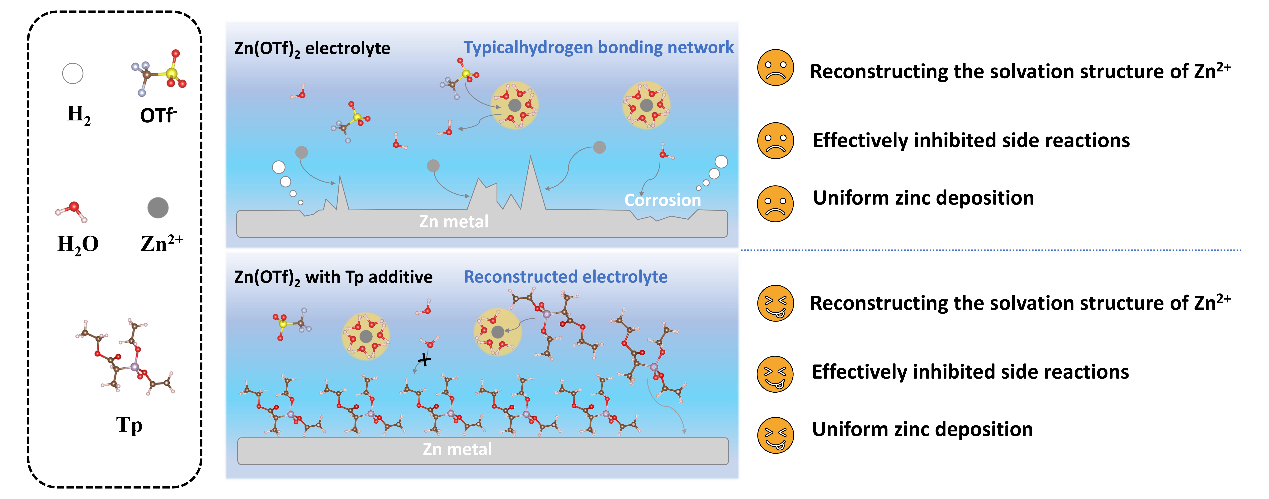
Theoretical schematic of Tp for initial electrolyte modification (Image by Prof. ZHANG's group)
Contact:
Prof. ZHANG Yining
Fujian Institute of Research on the Structure of Matter
Chinese Academy of Sciences
Email: ynzhang@fjirsm.ac.cn