Ammonia (NH3) is a crucial chemical raw material for modern industry and agricultural fertilizers, and is also serves as a significant hydrogen energy carrier, playing a vital role in advancing society's transition to sustainable energy. Photo-driven N2 reduction can be performed under ambient conditions, utilizing solar energy, making it a more energy-efficient and cleaner technology for ammonia synthesis.
However, due to the thermodynamic barrier caused by the high triple bond energy and the kinetic barrier caused by large energy gap, the problems from active site of inefficient N2 immobilization and activation emerged, which led to poor performance of the NH3 synthesis reaction by N2 reduction. Therefore, exploring photocatalysts and photocatalytic device for ammonia synthesis through N2 reduction under mild conditions is a great of significance.
In a study published in Angewandte Chemie International Edition, Prof. WANG Yaobing and his team from the Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, proposed using a proton-mediated photoelectrochemical device to separate N2 activation and N2 hydrogenation, thereby achieving photo-driven ammonia synthesis.
The researchers demonstrated the approach by using redox-catalysis covalent organic framework (COF) with a redox site (-C=O) for H+ reversible storage and a catalytic site (porphyrin Au) for N2 reduction reaction (NRR). Notably, the resulting COF as anode in the designed proton-mediated photoelectrochemical device can successfully store e- and H+ generated by hydrogen oxidation reaction (HOR) and achieved photo-driven NRR (108.97 umol g-1) under mild concentration. This strategy broadened the horizons of ammonia synthesis technology development, achieving solar energy conversion and ammonia production under green conditions.
The researchers also demonstrated that the TNAu-COF obtained by 1,4,5,8-naphthalenetetracarboxylic dianhydride (NTCDA) and porphyrin Au as building blocks exhibited efficient intramolecular charge separation efficiency of 935 picoseconds by femtosecond transient absorption (fs-TA) spectra. In the designed proton-mediated COF||Pt photoelectrochemical device, HOR process and redox reactions of functional groups within COF enabled the reversible storage of protons, providing a hydrogen source for the hydrogenation of N2 adsorbed and activated at Au catalytic active sites.
Remarkably, the H-bond network between the reduced COF (COF-H) and N2 adsorbed on Au promoted electron transfer from Au center to N2 and the N2 hydrogenation, achieving the continuous ammonia synthesis by the photoelectrochemical device.
In-situ FTIR results showed that the absorption peaks of N2 hydrogenation intermediates gradually increased with the illumination time increasing, and meanwhile, the -OH peak gradually weakened, accompanied by the increased -C=O peak. It indicated that the H from -OH on NTCDA in COF-H participated and promoted the photo-driven NRR.
In addition, theoretical calculation results further demonstrated that the intramolecular protons participated in the photo-driven NRR process and completed the first hydrogenation, achieving continuous hydrogenation processes and the following ammonia synthesis.
This study sparks new thought on artificial photosynthesis and provided new ideas for the design and implementation of proton-mediated photochemical ammonia synthesis systems in the future.
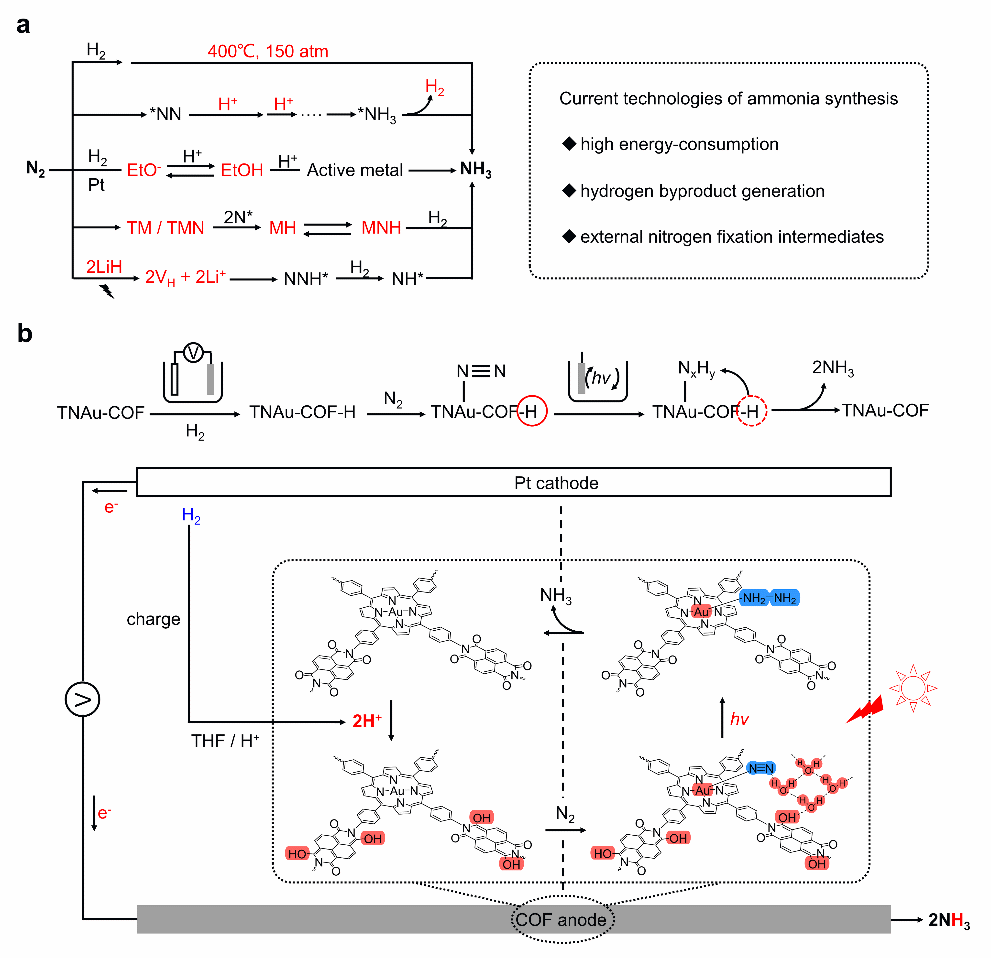
Schematic representation illustrating the photo-driven N2 reduction to NH3 via a proton-mediated COF||Pt photoelecrochemical device. (Image by Prof. WANG’s group)
Contact:
Prof. WANG Yaobing
Fujian Institute of Research on the Structure of Matter
Chinese Academy of Sciences
Email: wangyb@fjirsm.ac.cn