The oxygen reduction reaction (ORR) is a key process in energy conversion devices such as fuel cells and metal-air batteries. However, the widely used platinum-based catalysts face issues such as high cost and resource scarcity, which severely limit their large-scale application. Therefore, the development of efficient, inexpensive, and stable non-metallic catalysts has become a research hotspot. In recent years, high-entropy materials, with their unique multi-element doping characteristics and synergistic effects, have shown great potential in the field of catalysis.
In a study published in Angewandte Chemie International Edition, Prof. WEN Zhenhai from Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, has presented the first proof-of-concept of high-entropy engineered nanocarbons (HENCs) co-doped with five nonmetal elements (B, F, P, S, and N), which highlights the potential of high-entropy nonmental doping in revolutionizing electrocatalysis and related fields.
The researchers developed a reliable method for preparing HENC by employing in-situ polymerization modification and pyrolysis techniques using ZIF-8 metal-organic frameworks as precursors. This method incorporated five nonmetallic elements, namely B, F, P, S, and N, which significantly increased the system disorder and enhanced ORR catalytic activity. The resulting material exhibited activity comparable to commercial Pt/C (with a half-wave potential of 0.851 V) and demonstrated superior cycling stability.
They also provided experimental validation of the HENCs in practical applications by demonstrating their outstanding performance as cathode catalysts in zinc-air batteries with a peak power density of 604 mW cm−2 and long-term stability over 16 days, outperforming commercial Pt/C catalysts (542 mW cm−2). These findings highlighted the significant promise of HENCs for energy storage technologies.
The researchers’ systematic studies, which combined simulations with all-site calculations, revealed that the rich heteroatom activity and synergistic effects in HENCs promoted the formation of *O2 intermediates. N, P, and S were identified as the main active elements, while B and F enhanced system stability and mitigated the stripping effect of P.
This study offers a novel perspective on the use of high entropy doping in catalysis and opens new avenues for the development of cost-effective, high-performance electrocatalysts. The combination of heteroatom doping and high-entropy principles offers a promising strategy for designing next-generation electrocatalysts with broad applicability.
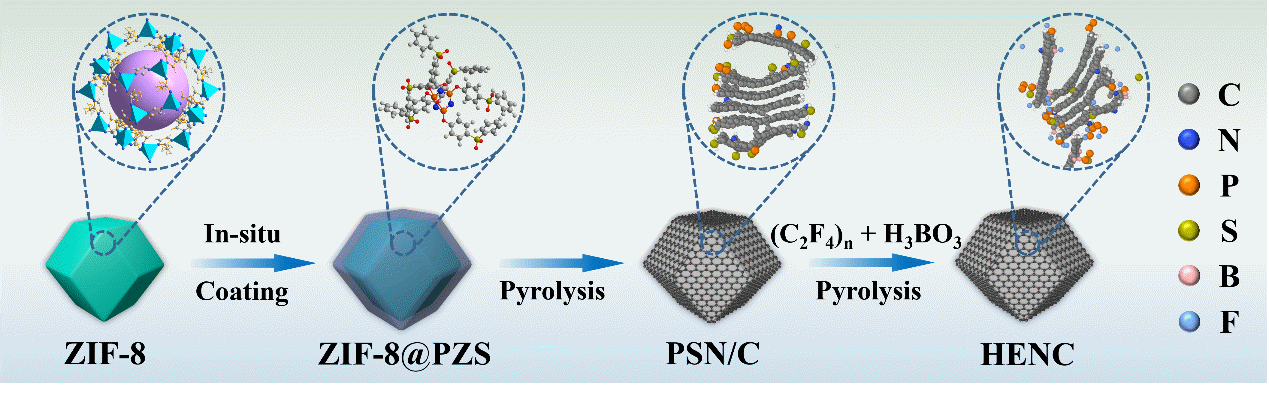
Schematic illustration of the synthesis of HENC (Image by Prof. WEN’s group)
Contact:
Prof. WEN Zhenhai
Fujian Institute of Research on the Structure of Matter
Chinese Academy of Sciences
Email: wen@fjirsm.ac.cn