As an important branch of porous organic cages, chiral porous organic molecular cages (CPOCs) have broad application prospects in the fields of chiral recognition, enantioselective separation and asymmetric catalysis. Therefore, the development of new chiral structural units for the construction of complex chiral cage structures is importantly critical and challenging.
Most reported CPOCs are typically synthesized using convergent chiral cyclohexanediamine or chiral binaphthyldiamine. Although concave-shaped calixarenes have been established as versatile building blocks for the synthesis of cage compounds, there are no reports on cages constructed from chiral calix[4]arene derivatives.
In a study published in J. Am. Chem. Soc., Prof. YUAN Daqiang, Prof. SU Kongzhao, and their colleagues from Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, introduced a gram-scale preparation method for a novel member of the family of chiral calix[4]arenes, phenol[4]arene (PC[4]A), which is then functionalized with tetraformyl groups to construct CPOCs using polyamine synthons.
(±)-PC[4]A can be synthesized from rccc-2,8,14,20-tetramethylresorcin[4]arene. The researchers introduced (1S)-(−)-camphoric acid chloride as an effective chiral auxiliary for obtaining enantiopure PC[4]A via simple column chromatography, yielding both enantiopure (+)-(P)-PC[4]A and (−)-(M)-PC[4]A (>99.9% enantiomeric excess) in 96% and 92% yields, respectively. The obtained materials were characterized by circular dichroism (CD) spectroscopy, Nuclear Magnetic Resonance (NMR), Fourier Transform infrared spectroscopy (FT-IR) and High Performance Liquid Chromatography (HPLC), while single-crystal X-ray diffraction was used to determine the absolute configurations of the chiral PC[4]A.
The (+)-(P)-PC[4]A and (−)-(M)-PC[4]A were further modified by formylation via the Duff reaction, resulting in the corresponding tetraformyl derivatives, (+)-(P)-PC[4]ACHO and (−)-(M)-PC[4]ACHO, with enantiomeric excess values exceeding 99.9%. Next, the researchers assembled the these as building blocks with two fluorescent amine synthesizers, such as bis(4-aminophenyl)benzene (BAP) and tris(4-aminophenyl)amine (TAP), to obtain a pair of [2 + 4] lantern-like CPOC-P1/M1 and [6 + 8] truncated octahedral CPOC- P2/M2, respectively.
The researchers used a series of fundamental characterizations such as NMR, Mass Spectrometry (MS), FT-IR, Ultraviolet–visible spectroscopy (UV-vis), CD spectroscopy, single-crystal X-ray diffraction, N2 adsorption, and Thermogravimetric Analysis (TGA) to validate the successful construction as well as the chirality, porosity, and stability of the CPOCs.
Additionally, the chiral recognition properties of these CPOCs are presented, demonstrating their ability to enantioselectively recognize amino acids and their derivatives. It is evident that P-configured CPOCs exhibit a preference for L-configured amino acid substrates compared to D-configured ones. These findings strongly suggest that CPOCs can be promising probes for the recognition of enantiomerically pure amino acids.
This study establishes a new member of chiral units, namely PC[4]A, for the design and construction of CPOCs with chiral recognition capabilities.
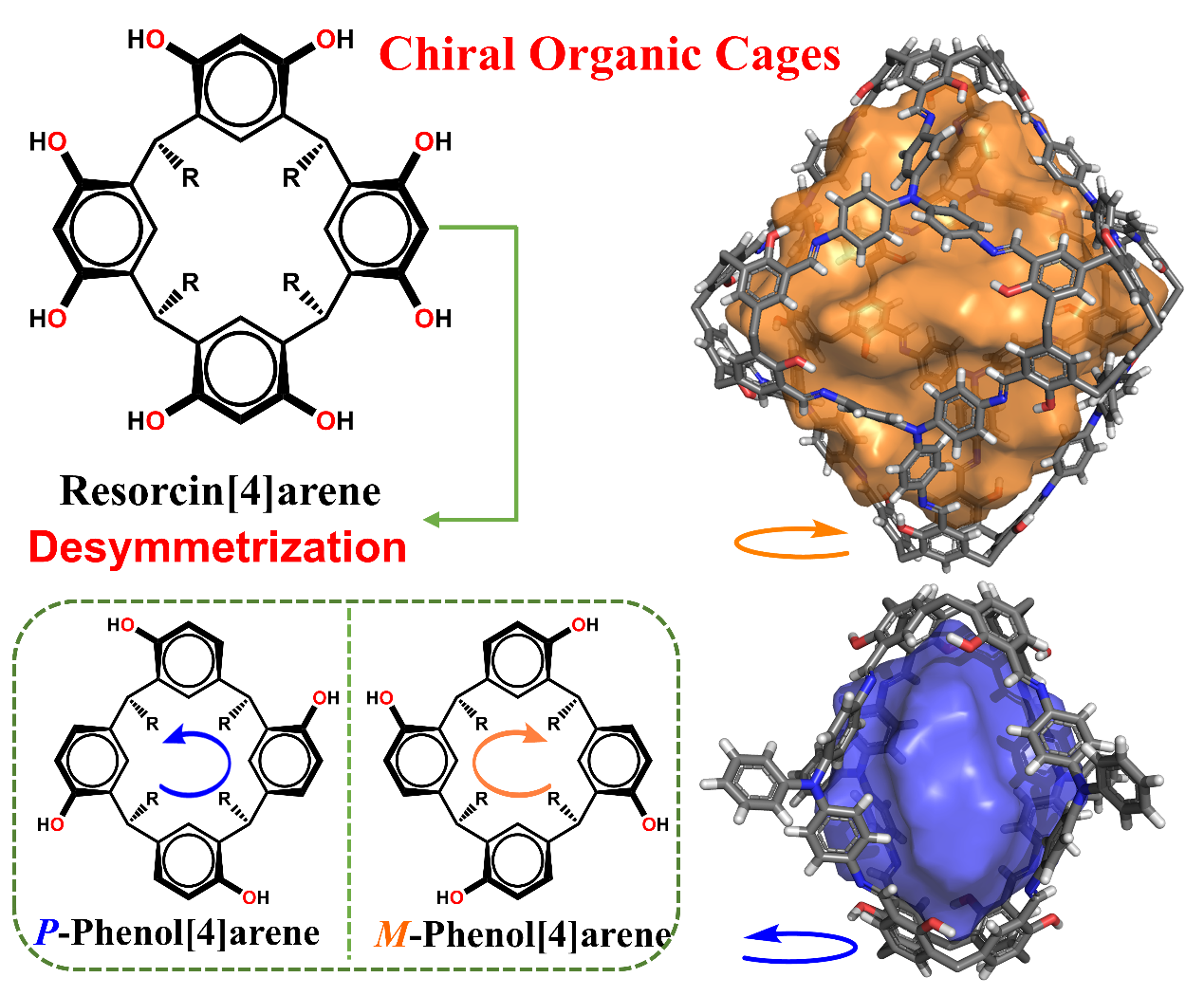
Schematic representation illustrating the PC[4]A-based chiral porous organic cages for amino acid capture and sensing. (Image by Prof. YUAN’s group)
Contact:
Prof. YUAN Daqiang
Fujian Institute of Research on the Structure of Matter
Chinese Academy of Sciences
Email: ydq@fjirsm.ac.cn