The quest for sustainable chemical processes has driven researchers to explore innovative catalysts for converting carbon monoxide into value-added products. Among these, dimethyl carbonate (DMC)—a green solvent and lithium-ion battery electrolyte—holds immense industrial promise. However, its synthesis via oxidative carbonylation of methyl nitrite (MN) faces a critical challenge: overcoming thermodynamic favorability toward dimethyl oxalate (DMO), a competing byproduct.
In a study published in Chemistry – A European Journal, a team led by Prof. GUO Guocong from Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, has decoded the synergistic role of palladium (Pd) centers and Lewis acid sites in steering selectivity toward DMC. By designing two model catalysts—Pd₁@NaY (with Pd-Lewis acid synergy) and Pd₁@T-NaY (isolated Pd)—the researchers unraveled a cooperative mechanism that could optimize industrial DMC production.
The team synthesized Pd₁@NaY by anchoring isolated Pd²⁺ atoms onto NaY zeolite, where adjacent Al³⁺ Lewis acid sites enhance catalytic activity. A second catalyst, Pd₁@T-NaY, was modified with a silica shell to deactivate Lewis acid sites, serving as a control. Advanced characterization (TEM, EXAFS, XPS) confirmed the atomic dispersion of Pd²⁺ in both catalysts, while IR-pyridine tests verified the selective removal of Lewis acidity in Pd₁@T-NaY.
Under optimized conditions (120°C, 0.1 MPa), Pd₁@NaY achieved 100% selectivity for DMC with minimal DMO or MN decomposition byproducts (e.g., dimethoxymethane). In contrast, Pd₁@T-NaY showed reduced activity and selectivity, highlighting the indispensable role of Lewis acid sites in stabilizing reactive intermediates. In situ DRIFTS experiments revealed that Al³⁺ sites in Pd₁@NaY activate MN and facilitate the transfer of *OCH₃ species—a critical intermediate for DMC formation—while their absence in Pd₁@T-NaY led to inefficient reactant activation.
Density functional theory (DFT) calculations illuminated the energy landscape: The synergy between Pd and Al³⁺ sites lowered energy barriers for C–O bond formation (0.03–0.20 eV), enabling rapid DMC synthesis, however Isolated Pd centers faced higher barriers (0.38–0.59 eV), favoring sluggish kinetics and side reactions. The study further visualized how Al³⁺ dynamically stabilizes *OCH₃ within the zeolite framework, ensuring efficient mass transfer and product desorption.
This study not only clarifies the molecular mechanism of Pd-Lewis acid cooperation but also provides a blueprint for designing next-generation catalysts. Future efforts will focus on optimizing Pd/Al³⁺ ratios to suppress MN decomposition while maximizing DMC yield—a critical step for scalable production.
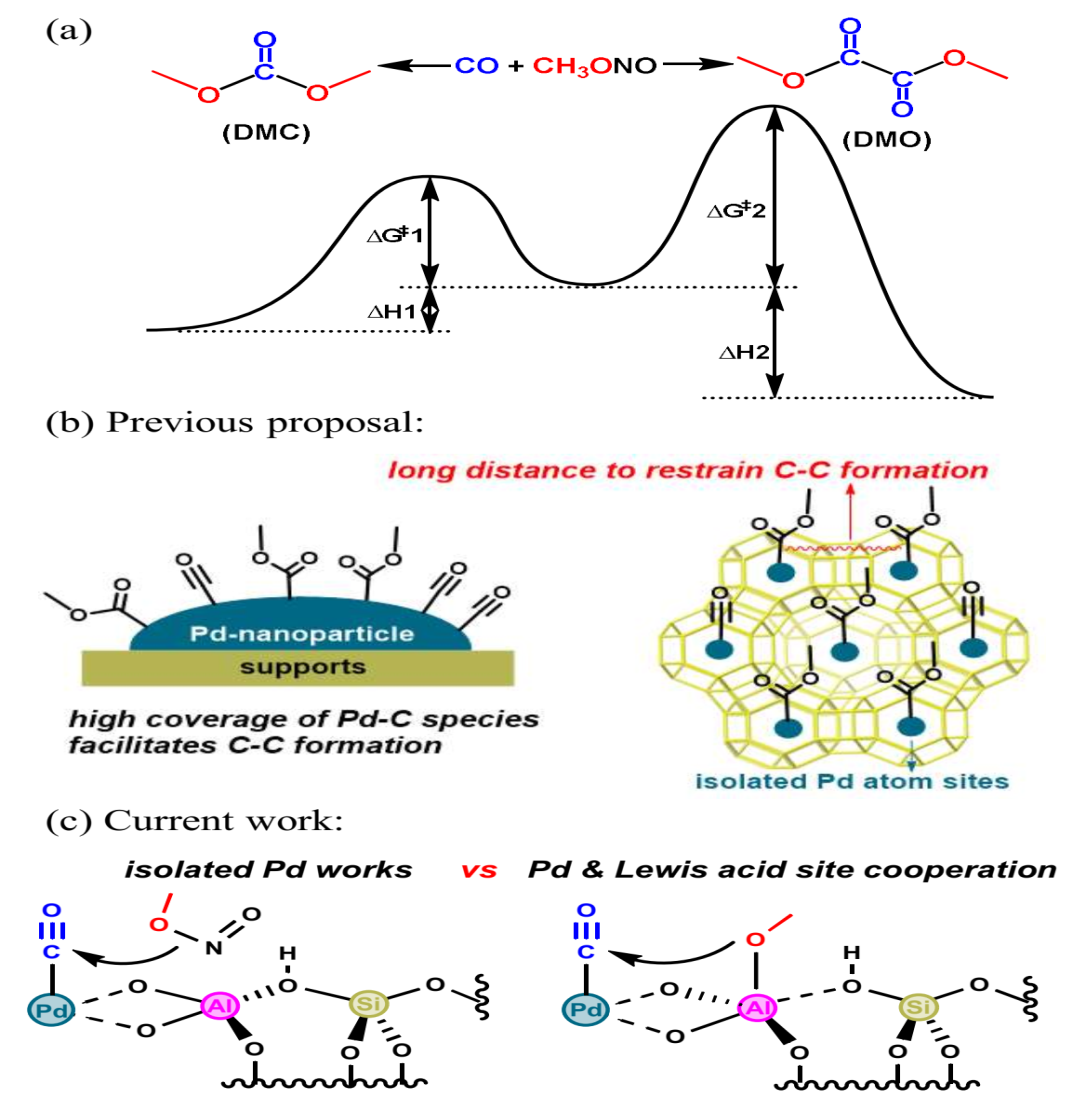
Chemo-selectivity for MN carbonylation reaction and the proposed mechanism for DMC production. (Image by Prof. GUO’s group)
Contact:
Dr. LIN Shujuan
Fujian Institute of Research on the Structure of Matter
Chinese Academy of Sciences
Email: sjlin@fjirsm.ac.cn