Metal-organic framework (MOF) materials have considerable potential for practical applications in solid-phase separation technology. However, the application of MOFs for zirconium and hafnium separation remains unexplored.
In a study published in ACS Applied Materials & Interfaces, the research group led by Prof. YANG Fan from Fujian Institute of Research on the Structure of Matter of the Chinese Academy of Sciences pioneered the application of metal-organic framework (MOF) materials in the field of zirconium and hafnium separation.By constructing a MIL-101-diglycolic acid (DGA) (MOF) coupling with betaine hydrochloride system, the researchers addressed the issues associated with traditional zirconium and hafnium separation methods, which require the use of environmentally hazardous HSCN and highly acidic conditions. This innovation enables green and efficient zirconium-hafnium separation under low-acid conditions.
The researchers employed MIL-101-NH2 as the matrix material and synthesized MIL-101-DGA by incorporating DGA functional groups through a single-step ring-opening reaction. This modification endows the MOF with tridentate coordination ability and excellent binding affinity for zirconium ions. Additionally, betaine hydrochloride was introduced as a competitive ligand that preferentially forms water-soluble complexes with hafnium ions. This strategy establishes a "push-pull" mechanism, thereby significantly improving the efficiency of zirconium and hafnium separation.
The MIL-101-DGA coupling betaine hydrochloride system achieves efficient separation of zirconium and hafnium under low acid conditions. At a pH of 0.50, the separation factor (βZr/Hf) reaches 19.7, and at a pH of 1.46, the separation factor (βZr/Hf) is 8.2.
The researchers introduced L-aspartic acid as a desorption enhancer. In a traditional single sulfuric acid system, the desorption of zirconium and hafnium was difficult. However, by combining 0.2 mol/L L-aspartic acid with 0.2 mol/L sulfuric acid, the desorption efficiencies of zirconium and hafnium were significantly increased to 87% and 90%, respectively. This approach achieved efficient desorption of zirconium and hafnium at low acid concentrations.
The MIL-101-DGA material demonstrates excellent cycling and immersion stability, with a loss of physicochemical properties of less than 7% after multiple adsorption/desorption cycles. After immersion for 7 days in betaine hydrochloride solutions with pH values ranging from 0.50 to 2.00, as well as in a mixed solution of 0.2 mol/L L-aspartic acid and 0.2 mol/L sulfuric acid, the material demonstrated excellent stability in the separation environment.
This study not only addresses the high toxicity and high acidity issues of traditional separation methods but also broadens the scope of solid-phase separation materials, paving the way for the application of MOFs in the field of separation.
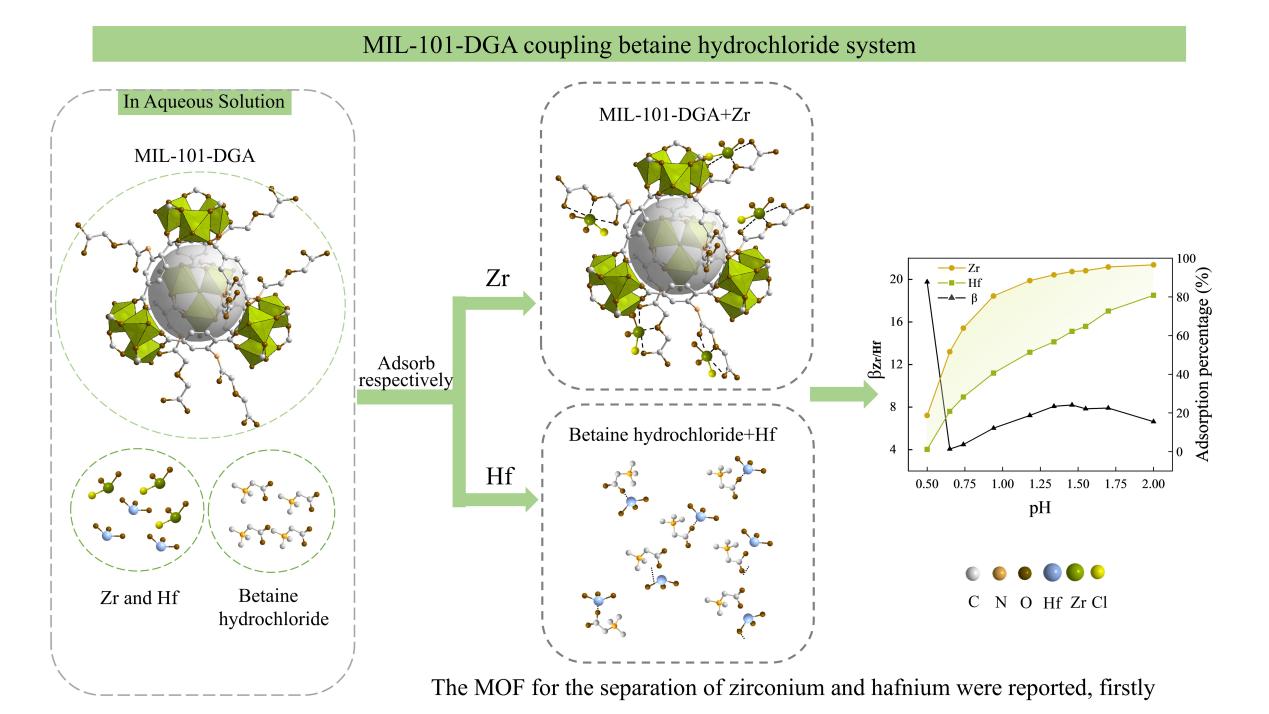
Separation of zirconium and hafnium by MIL-101-DGA coupling betaine hydrochloride system (Image by Prof. YANG's group)
Contact:
Prof. YANG Fan
Fujian Institute of Research on the Structure of Matter
Chinese Academy of Sciences
Email: fanyang2013@fjirsm.ac.cn