Cancer therapy often faces challenges such as drug resistance and immune evasion. Conventional therapeutic antibodies for immune anti-cancer therapy have shown shortcomings like high immunogenicity and lacking capability of enabling efficient intracellular delivery. PD-L1, an immune checkpoint inhibitor, and VEGFR2, essential for cancer metastasis, play pivotal roles in tumorigenesis. However, their miniature bispecific intracellular nanobodies for combining check-point blockade and anti-metastasis anticancer therapy remain underexplored.
In a study published in Molecular Cancer, Professor ZHANG Lei and Professor CHEN Zhuo (team leader) from the Fujian Institute of Research on the Structure of Matter, along with their collaborator, Professor KE Zunfu from The First Affiliated Hospital of Sun Yat-sen University, reported a novel bispecific nanobody, FAP1V2, designed to revolutionize cancer treatment by simultaneously targeting two critical pathways: immune checkpoint blockade and metastasis suppression. The study explored the mechanisms involved in how transient intracellular expression of PD-L1 and VEGFR2 bispecific nanobody in cancer cells inspired long-term T cell activation and infiltration to combat tumor and inhibit cancer metastasis.
The researchers found that the newly developed FAP1V2 nanobody contains the heavy chain variable regions VH of antibodies against PD-L1, an immune checkpoint protein, and VEGFR2, a key driver of angiogenesis and metastasis. Lacking the Fc fragment, FAP1V2 had reduced immunogenicity and enabling efficient intracellular delivery, whereas kept highly specific and efficient ability to bind and block the antigens PD-L1 and VEGFR2.
In preclinical models, two rounds of transient FAP1V2 expression in Lewis lung carcinoma (LLC) cells resulted in remarkable tumor suppression. Notably, 1 out of 6 treated mice exhibited complete eradication of secondary tumors. The therapy enhanced T cell infiltration into tumors, reduced PD-1high immune cells (associated with T cell exhaustion), and boosted CD25high activated T cells in the spleen, indicating robust systemic immune activation. Additionally, FAP1V2 significantly inhibited cancer cell migration by blocking VEGFR2 signaling, preventing metastasis to the lung and liver.
Transcriptome analysis revealed that FAP1V2 modulates critical pathways, including Wnt/β-catenin and PI3K/AKT, while downregulating transcription of metastasis-promoting genes like MET and MMPs. This dual-action mechanism—enhancing immune response while stifling tumor spread—positions FAP1V2 as a transformative candidate for combination cancer therapies. With its high biocompatibility and targeted approach, FAP1V2 offers hope for overcoming traditional limitations in cancer treatment, paving the way for more effective and personalized therapies.
This study presents a potential strategy for enhancing immune activation while inhibiting metastasis in tumor therapy. It introduces a new framework for investigating the intracellular regulation of signaling pathways through the application of VH intrabodies.
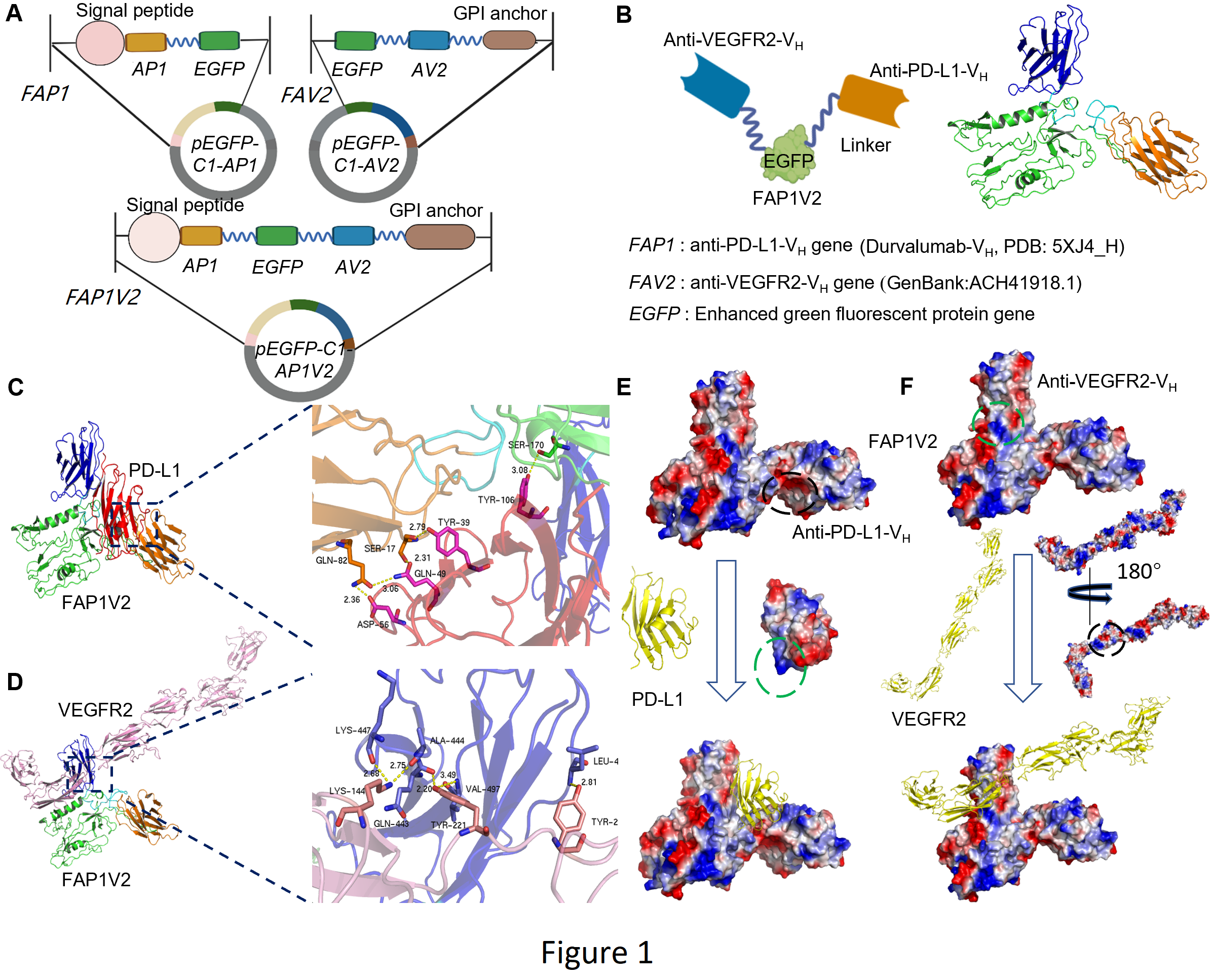
Design of the intrabodies and molecular docking of FAP1/PD-L1 or FAP1V2/VEGFR2 complex. (Image by Prof. CHEN’s group)
Contact:
Prof. ZHANG Lei
Fujian Institute of Research on the Structure of Matter
Chinese Academy of Sciences
Email: zhangl@fjirsm.ac.cn