Aqueous zinc-ion batteries (AZIBs) offer high capacity (820 mAh g-1), low redox potential (−0.76 V vs. SHE), low cost, and eco-friendliness for large-scale storage. However, anode-electrolyte interface instability causes corrosion and dendrite growth. Unstable interfaces accelerate inhomogeneous Zn2+ deposition, triggering ion gradient shifts and local depletion that exacerbate dendrites.
Concurrently, solvated water molecules adsorb onto Zn surfaces, promoting the hydrogen evolution reaction (HER). HER generates H2, corrodes zinc, and forms insulating by-products. These issues collectively degrade plating uniformity, deteriorating cycling performance. Suppressing dendrites and HER is thus critical for AZIB commercialization.
In a study published in Journal of Colloid and Interface Science, the research group led by Professor ZHANG Yining from Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, has reported a self-adsorbing electrolyte additive-7-(2,3-Dihydroxypropyl) theophylline (DHTP). When added to the electrolyte, this additive can stabilize the zinc anode and enhance the cycle life of aqueous zinc-ion batteries (AZIBs).
Theoretical calculations combined with experimental characterization demonstrate that DHTP molecules can preferentially adsorb on the zinc anode. On the one hand, this reduces the contact between water molecules and the zinc anode, minimizing the occurrence of side reactions such as hydrogen evolution and corrosion. On the other hand, due to the different adsorption energies of DHTP on different crystal planes, the Zn (002) crystal plane will be preferentially exposed, and the zinc foil will grow along the (002) plane. This effectively suppresses dendrite growth and achieves a stable zinc anode.
Benefiting from these advantages, compared with the benchmark Zn(CF3SO3)2 electrolyte, after adding the DHTP additive, the Zn||Zn symmetric cell can cycle for more than 800 hours at a current density and areal density of 1 mA cm-2 and 1 mA h cm-2, and the Zn||Cu cell can maintain a Coulombic Efficiency (CE) of nearly 100% and cycle for more than 200 cycles. For the Zn||NaV3O8·1.5H2O full cell, it can also cycle 9000 times at a high current density of 10 A g-1 with a capacity retention rate as high as 89%.
This molecular adsorption strategy based on DHTP provides a novel approach to constructing highly reversible zinc anodes for advanced AZIBs.
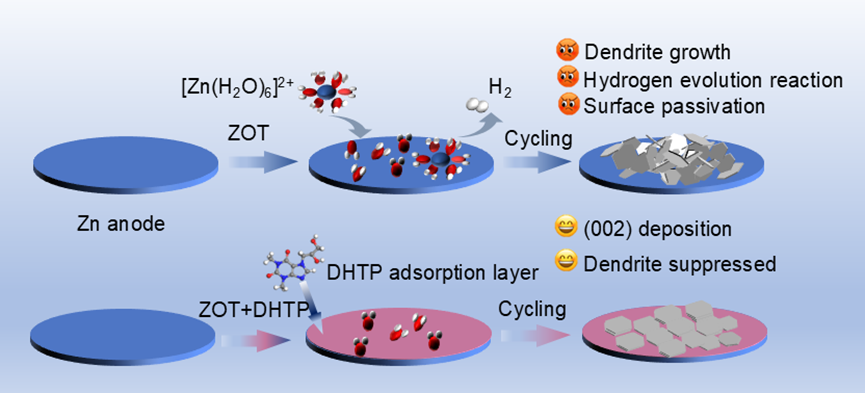
Schematic diagram of the mechanism of DHTP (Image by Prof. ZHANG Yining's group)
Contact:
Prof. ZHANG Yining
Fujian Institute of Research on the Structure of Matter,
Chinese Academy of Sciences
Email: ynzhang@fjirsm.ac.cn