Photoswitchable catalysis has long been constrained to simple reaction activation/deactivation ("on/off" mode), while achieving selective switching between distinct product pathways within a single catalyst remains a fundamental challenge. Metal-organic cages (MOCs) offer programmable microenvironments for tailored reactivity, yet existing light-responsive systems require multi-cage collaboration or structural reorganization.
In a study published in Angewandte Chemie International Edition, a research team led by Prof. SUN Qingfu from the Fujian Institute of Research on the Structure of Matter (FJIRSM), Chinese Academy of Sciences, has developed a viologen-functionalized europium tetrahedral cage (Eu₄L₆) that enables light-triggered switching between two divergent catalytic pathways within a single molecular architecture.
The researchers centered on a custom-designed redox-active ligand (L), which integrates viologen photoresponsive units with tridentate chelating groups. Through precise self-assembly with europium(III) triflate, the team constructed a tetrahedral Eu₄L₆ cage characterized by single-crystal X-ray diffraction. The structure reveals six viologen units spatially isolated at the cage edges—confirmed by EPR spectroscopy—which critically prevent radical dimerization and enable efficient photoinduced electron transfer.
Catalytic performance studies demonstrated unprecedented control: When exposed to 365 nm light, the cage catalyzes oxidative coupling of sodium tetraphenylborate (NaBPh₄), generating biphenyl (72% yield) and phenol (70%) through a radical-mediated pathway. Conversely, in darkness, the same cage switches to catalytic decomposition, producing mono-arenes like benzene (81% yield from tetrakis(4-methylphenyl)borate). This divergent synthesis arises from light-modulated energy barriers—validated by DFT calculations—where illumination enables the system to overcome the oxidative coupling barrier, while in darkness, catalytic decomposition proceeds spontaneously yet slowly through a near-thermoneutral pathway.
Mechanistic evidence emerged through multifaceted characterization: UV-vis spectroscopy identified a 344 nm charge-transfer band upon substrate binding, while Stern-Volmer quenching confirmed efficient photoinduced electron transfer. EPR trapping experiments with DMPO captured superoxide radical intermediates (O₂•⁻) during both pathways.
This study establishes the first paradigm for photoswitchable divergent synthesis within a single-cage system, overcoming traditional limitations in photocatalyst design. By enabling precise product selectivity through light/dark transitions, it opens avenues for adaptive synthetic systems, smart nanoreactors, and artificial photosynthetic devices.
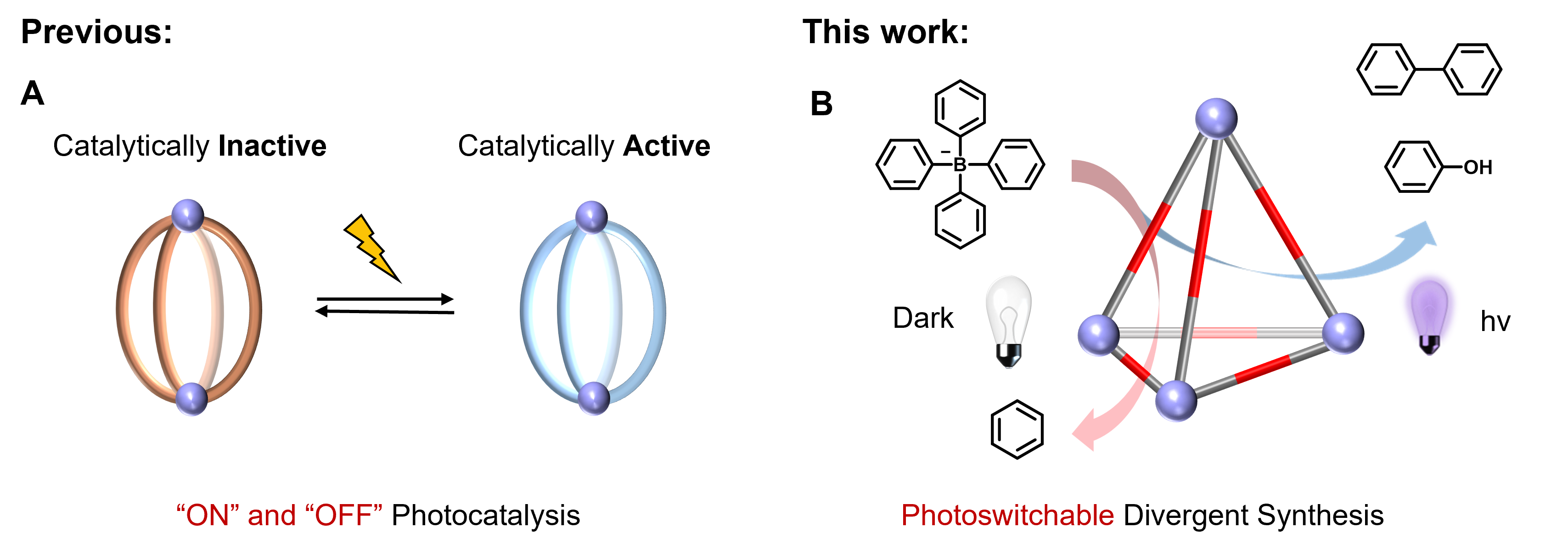
Schematic diagram of photoswitchable divergent synthesis in molecular cages(Image by Prof. SUN’s group)
Contact:
Prof. SUN Qingfu
Fujian Institute of Research on the Structure of Matter
Chinese Academy of Sciences
Email:qfsun@fjirsm.ac.cn