Hydrogen peroxide (H2O2) is a versatile green oxidant with extensive applications in various industrial processes, including wastewater treatment, paper bleaching, and chemical synthesis. However, the conventional anthraquinone method for H2O2 production is energy-intensive and involves complex operational procedures.
In recent years, the photo-driven two-electron oxygen reduction reaction (2e− ORR) has emerged as a promising alternative pathway for H2O2 synthesis due to its environmental friendliness and flexibility. Among various photocatalysts, covalent organic frameworks (COFs) have garnered significant attention for their exceptional light-harvesting properties and programmable structures. Nevertheless, the photocatalytic efficiency of COFs in H2O2 production has been constrained by rapid charge recombination and undefined electron transfer pathways caused by redundant inactive sites around the catalytic centers.
In a study published in the Journal of the American Chemical Society, the research team led by Prof. CAO Rong from Fujian Institute of Research on the Structure of Matter of Chinese Academy of Sciences has reported a groundbreaking strategy that transforms the imine units in COFs from electronic recombination centers into active catalytic sites for the ORR.
The researchers synthesized three types of COFs with systematically tuned imine polarization: azine-linked COF (A-COF) with low polarization, imine-linked COF (I-COF) with medium polarization, and hydrazone-linked COF (H-COF) with high polarization. Through ultrafast spectroscopy and theoretical calculations, they demonstrated that reducing the polarization of imine units in A-COF could simultaneously suppress excited-state deactivation, enhance O2 adsorption and activation, and enable direct electron transfer from the triphenylbenzene photosensitizer units to the imine catalytic center via the proximity effect. This unique configuration minimized charge recombination and maximized electron utilization efficiency.
A-COF demonstrated exceptional performance in photocatalytic H2O2 production, achieving a yield of 2311 μmol g−1 in 2 hours under visible light. This yield is 3.8 and 2.9 times higher than those of I-COF and H-COF, respectively. The researchers attributed this superiority to A-COF's low imine polarization, which enhanced O2 adsorption via Pauling-type end-on bonding and stabilized the excited state for prolonged electron availability. The study also highlighted the critical role of spatial organization within COFs in optimizing electron transfer pathways and catalyst efficiency.
This study pioneers a molecular-level strategy to engineer catalytic sites in COFs through polarization control, transforming traditionally recombination-prone imine units into efficient active centers. It provides a generalizable blueprint for designing high-performance photocatalytic systems with optimized spatial and electronic structures. The findings not only advance the field of H2O2 photosynthesis but also offer valuable insights for developing efficient catalysts for other energy-related reactions.
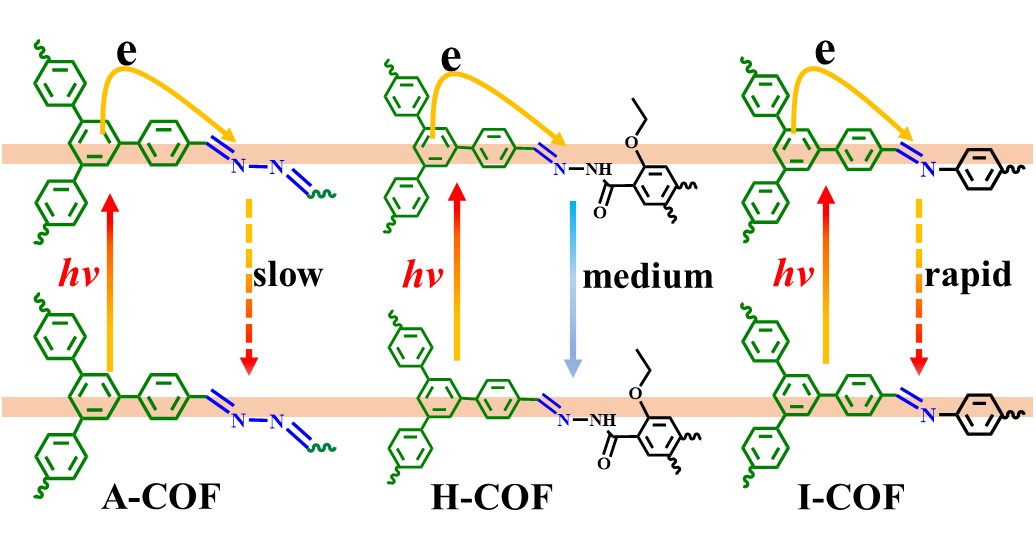
Comparision of excited state dynamics between A-COF, H-COF, and I-COF(Image by Prof. CAO's group)
Contact:
Prof. CAO Rong
Fujian Institute of Research on the Structure of Matter
Chinese Academy of Sciences
Email: rcao@fjirsm.ac.cn