Electrocatalytic reduction of CO2 has been long viewed as a promising approach for utilization of CO2 and reducing atmospheric greenhouse gas level. Transition metal complexes represent an important class of electrochemical CO2 reduction (eCO2RR) catalysts due to their atom-precise and tuneable active site structures. Synthesis of binuclear complexes introduces potential synergistic effects between metal centres, and construction of heterometallic sites allows electronic structure tuning of the active site, which may regulate reaction pathways and promote the formation of deep reduction products.
In a study published in Chemical Communications, Prof. ZHANG Teng and Prof. CAO Rong from the Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, reported the synthesis and eCO2RR performance of a series of homometallic and heterometallic complexes and elucidated the regulatory effect of heterometallic sites in eCO2RR performance.
The researchers synthesized three binuclear complexes, denoted as Cu2L2(ClO4)2, CuNiL2(ClO4)2, and CuCoL2(ClO4)2, and verified their structures by single crystal X-ray crystallography. All three complexes adopt in a planar binuclear coordination geometry with the moiety of CuMN4O2 (M = Cu, Ni, or Co). Electrochemical tests revealed that these complexes exhibited eCO2RR activity in acetonitrile solution. The homometallic complex Cu2L2(ClO4)2 produced formic acid selectively, while the heterometallic complexes exhibited moderate selectivity for hydrocarbon products. The Faradaic efficiencies (FEs) for methane and ethylene were found to be 8% and 9%, respectively, with CuCoL2(ClO4)2 catalyst.
Control experiments showed that the mononuclear analogue, CuL1, exhibited eCO2RR selectivity towards CO, indicating that production of formic acid is promoted by the binuclear structure. Enhanced selectivity for hydrocarbon products was attributed to synergistic electronic effect on the bimetallic Cu-Co site. Combination of kinetic isotope effect (KIE) measurements and density functional theory (DFT) calculations revealed a possible mechanism of eCO2RR. Differences of the CO adsorption energies on the reduced Cu site play a vital role on the higher hydrocarbon FEs on CuCoL2(ClO4)2.
With a precise control of the active site composition, this study provides a valid approach for the development of novel homogeneous eCO2RR catalysts and demonstrates that catalytic eCO2RR performance of metal complexes can be systematically controlled through introduction of different metals.
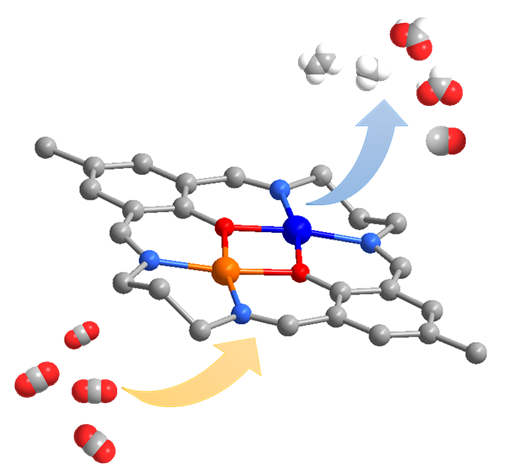
Illustration of the Research(Image by Prof. ZHANG’s group)
Contact:
Prof. ZHANG Teng
Fujian Institute of Research on the Structure of Matter
Chinese Academy of Sciences
Email: zhangteng@fjirsm.ac.cn