Spherical nanoparticles balance the high surface energy imposed by nanoscale dimensions against the surface energy minimization promoted by spherical geometry. Their surface properties are crucial to their application effectiveness. However, limited by their tiny size and structural uncertainties, traditional analytical methods struggle to obtain clear information on surface adsorption behavior.
Spherical nanoclusters with atomically precise structures, serving as molecular models, hold promise for solutions.Nevertheless, spherical clusters are difficult to design and synthesize due to a lack of effective strategies, and achieving a balance between stability and porosity in these discrete compounds in the solid state is also challenging.Compared to framework materials, their host-guest chemistry studies are mostly conducted in solution, making direct characterization using single-crystal x-ray diffraction difficult.
In a study published in Nature Synthesis, a research team led by Professor ZHANG Jian and Professor FANG Weihui from the Fujian Institute of Research on the Structure of Matter (FJIRSM), Chinese Academy of Sciences, drew inspiration from micellar self-assembly principles and proposed a "co-encapsulation" strategy. Through the synergistic guidance of flexible sterically hindered ligands and inorganic lone pair electrons, they successfully constructed the first spherical aluminum-oxo cluster (SAlOC-1).
The surface of SAlOC-1 contains a large number of monodentate ligands suspended on coordinatively unsaturated Al3+ ions, enabling it to simulate the complex surface environment of nanoparticles.Importantly, SAlOC-1 retains its spherical morphology which maximizes exposure of surface supramolecular sites, while exhibiting unusual low symmetry, which reduces disorder in crystallographic studies of guest molecules.
Researchers have discovered that SAlOC-1 can bind up to 20 guests across a wide volume range at room temperature through a single-crystal-to-single-crystal transformation. SAlOC-1 has unique advantages in guest recognition, overcoming the limitations of host-guest chemistry of traditional discrete system in solution, and is simple, rapid, and direct to operate. Furthermore, this surface host-guest chemistry is both universal and selective, and also exhibits biomimetic characteristics of multi-component combination. The theoretical calculations provided by Professor Chunsen Li's research group of the Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, revealed that the mechanism by which SAlOC-1 captures guests is significantly different from classic framework materials, mainly relying on dynamically rotatable monodentate ligands acting as "molecular catchers" on flexible surfaces, thereby reducing dependence of the diffusion mechanism on high porosity.
This study not only provides new insights into the precise construction of spherical aluminum-oxo clusters and the rational design of flexible surfaces, but also offers new avenues for the solid-state host-guest chemistry of discrete compounds, the structural identification of organic molecules, and the surface modification of nanoparticles.
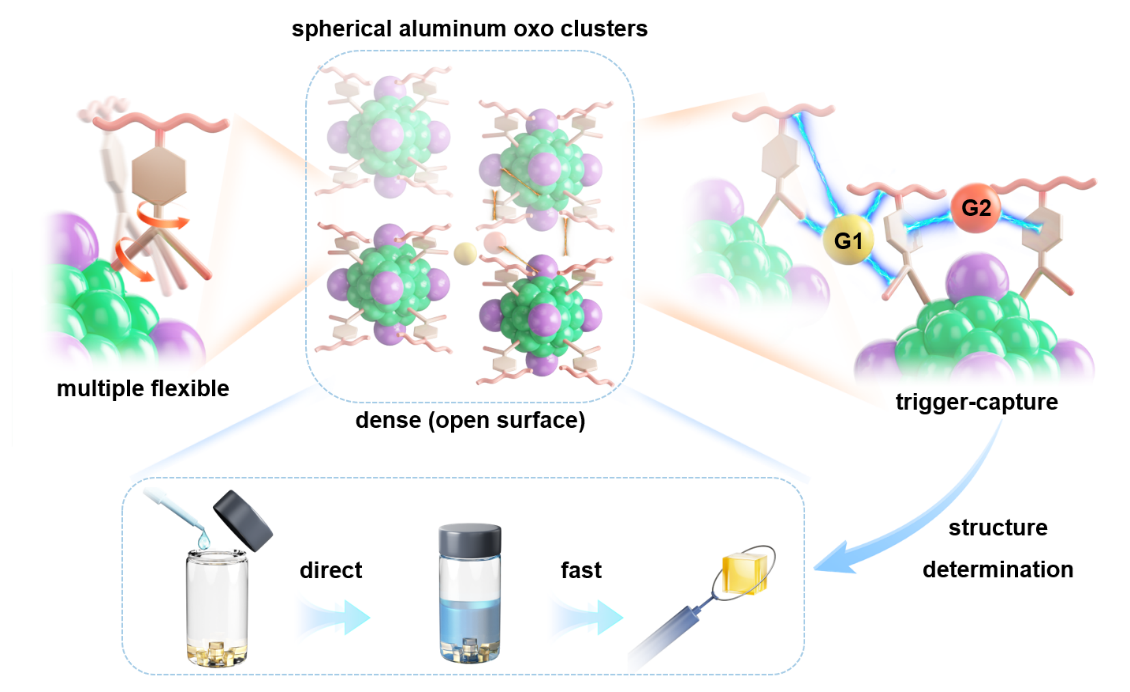
Schematic diagram of the host-guest chemistry on the surface of spherical aluminum-oxo clusters (SAlOC-1)(Image by Prof. FANG Weihui)
Contact:
Prof. FANG Weihui
Fujian Institute of Research on the Structure of Matter
Chinese Academy of Sciences
Email: fwh@fjirsm.ac.cn