Ammonia (NH3) is an essential chemical feedstock, yet its conventional production via the Haber–Bosch process is widely recognized as energy-intensive and carbon-emissive, motivating the search for greener synthesis routes.
Electrocatalytic nitrate reduction (NO3⁻RR) powered by renewable electricity has attracted attention because it can simultaneously produce NH₃. However, NO3⁻RR involves complex multistep deoxygenation/hydrogenation, suffers from sluggish kinetics and competing hydrogen evolution, and faces downstream bottlenecks in NH3 isolation and fixation for scalable deployment.
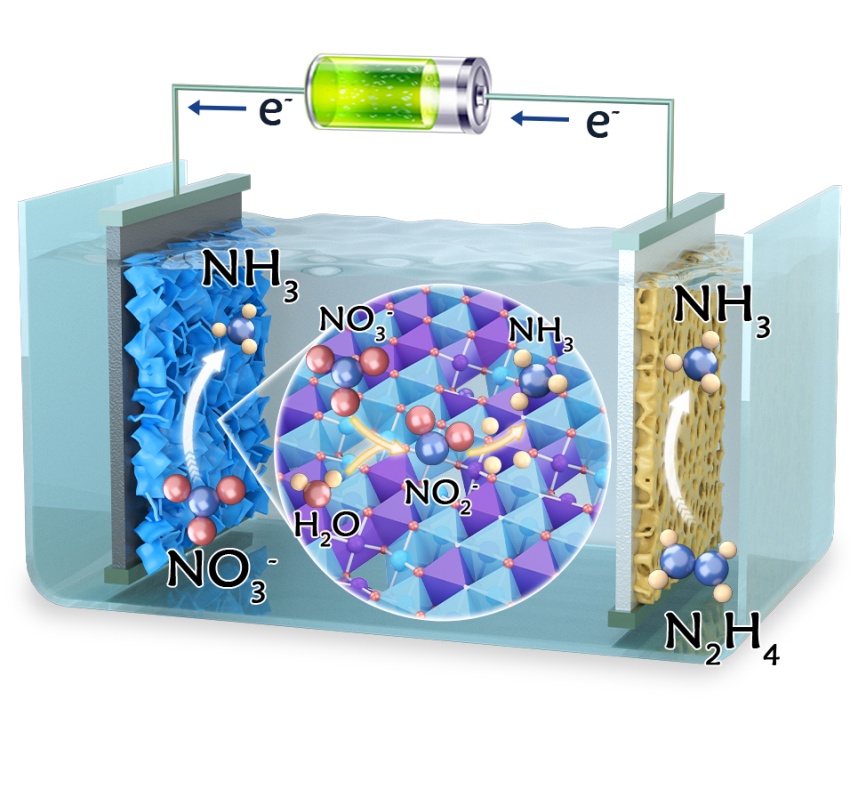
A research team led by Prof. ZHANG Linjie and Prof. HAN Lili from Fujian Institute of Research on the Structure of Matter (FJIRSM) of Chinese Academy of Sciences, reported a surface-reconstructed NiFe hydroxide (NiFe-LDH-R) that drives high-rate NO3⁻-to-NH3 conversion and further established a membrane-free bipolar electrosynthesis configuration that delivers Faradaic efficiency (FE) exceeding 100%. In addition, the researchers designed a workflow to continuously capture the electrosynthesized NH₃ and upgrade it into methenamine, achieving gram-scale product (4.1 g). This study was published in Angew. Chem. Int. Ed.
The researchers synthesized nanosheet superstructures of nickel-iron layered double hydroxide (NiFe-LDH) that were grown directly on an iron foam substrate through a one-step in situ self-corrosion process at room temperature. This enabled the fabrication of large-area supports on a laboratory scale. The as-prepared NiFe-LDH was then cathodically reconstructed to form NiFe-LDH-R, in which oxygen-vacancy (Ov) clusters preferentially localize around low-valence Ni sites.
The resultant restructured NiFe-LDH (NiFe-LDH-R) demonstrates excellent concentration-universal NH3 electrosynthesis activity in 1 M KOH, notably sustaining high Faradaic efficiencies (FEs, 88.5%–95%) across a broad potential range and attaining an ampere-level current density (–1.46 A cm–2) together with a remarkable yield rate of 104.1 mgNH3 h−1 cm−2.
In-situ spectroscopic analyses revealed boosted hydrogenation kinetics and a thermodynamically favorable NOH pathway for NiFe-LDH-R, which is further decoded by theoretical calculations indicating that synergized Ov/Fe and low-valence Ni sites respectively enhance NO3– adsorption and directional active hydrogen (*H) supply, thus streamlining overall energy barriers.
Moreover, the researchers established a new-style membrane-free bipolar electrosynthesis system, which enables unprecedent NH3 FEs exceeding 100% and scalable NH3 valorization into 4.1 g of methenamine.
This study rekindles power of electrochemical restructuring in catalyst advance and pioneers a new paradigm for energy-efficient electrochemical NH3 production and fixation.
Comparision of excited state dynamics between A-COF, H-COF, and I-COF(Image by Prof. HAN's group)
Contact:
Prof. HAN LiLi
Fujian Institute of Research on the Structure of Matter
Chinese Academy of Sciences
Email: llhan@fjirsm.ac.cn