Hydrogen-bonded organic frameworks (HOFs) are crystalline porous materials self-assembled via intermolecular hydrogen bonds. Their reversible nature attracts attention in catalysis, separation, and medicine. Pore sizes from angstroms to nanometers are feasible by designing building blocks, but larger pores pose synthetic challenges, increasing cost, toxicity, and instability. The template method offers an alternative, overcoming constraints of molecular design. This enables macroporous HOFs from small molecules, easing fabrication, expanding the HOF library to low-toxic options, and enhancing clinical potential. High-density hydrogen bonds in the structure resolve the porosity-stability conflict, yielding robust macroporous HOFs.
In a study published in Nature Communications, the research team led by Prof. LIU Tianfu from Fujian Institute of Research on the Structure of Matter of Chinese Academy of Sciences has reported a promising strategy that facilitates the fabrication of highly ordered materials in single-crystal form with high physiological stability, and enhanced mass transfer. Importantly, the strategy greatly broadens the HOF library to small, affordable,low-toxic, and clinically applicable molecules, making HOFs promising biocompatible porous substrates for bio-related applicationssuch as enzyme immobilization.
The researchers selected an innovative approach for fabricating highly ordered macroporous HOFs in single-crystal form (denoted by OM-HOFs) based on five small, affordable, clinically applicable organic molecules, melamine (MA), trimesic acid (TMA), trihy-droxybenzene (THB), methylene blue (MB) and uric acid (UA), through template method. The multiple hydrogen bonds and extensive π–π interaction between molecules guide the self-assembly in avoidance of the interference from template and endow the assemblies with robustness to survive upon template removal.
As a result, highly ordered macroporous HOFs in single-crystal form (denoted by OM-HOFs) were successfully synthesized, whose structure can be unambiguous determined through electron-diffraction analysis. Such a type of macroporous material based on biocompatible molecules emerge as promising candidates for enzyme immobilization with enhanced stability and recyclability. The study demonstrated that Try@OM-PFC, employed as a cellular scaffold, can promote fibrocyte differentiation, offering the advantagesof transparency for observation, shape adaptability, and moisture retention.
This study presents the synthesis of a series of highly ordered macroporous HOFs in single crystal form based on small biocompatible and clinically applicable molecules through template method.
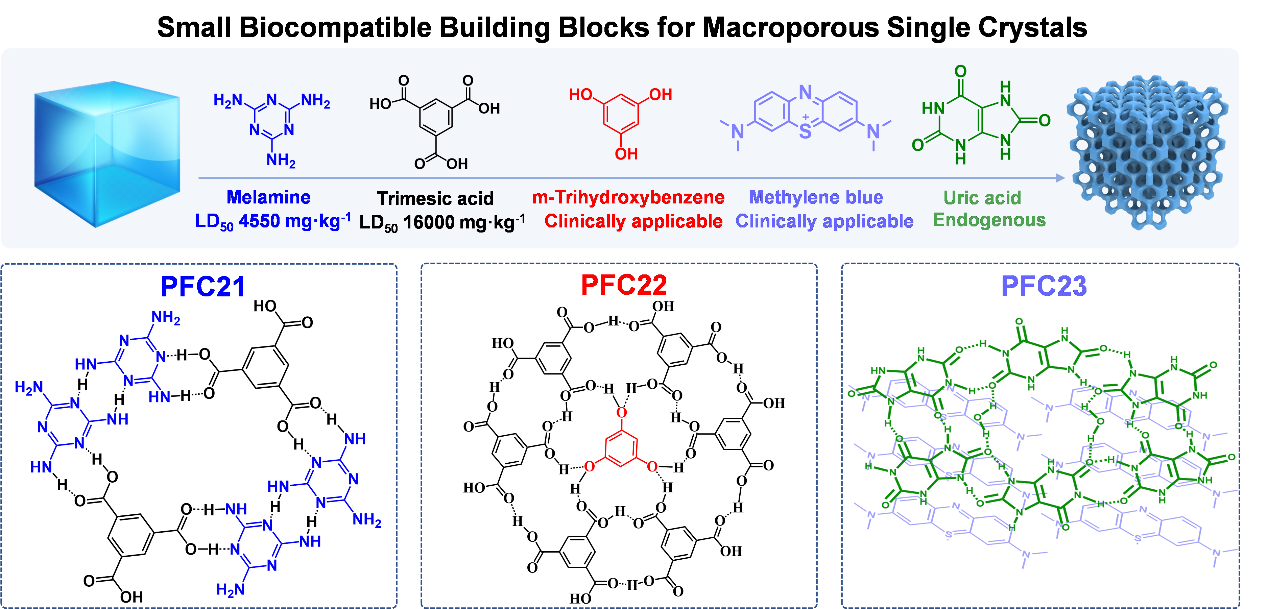
Schematic illustration of the self-assembly process of OM-HOFs (OM-PFC21, OM-PFC22, and OM-PFC23). (Image by Prof. LIU's group)
Contact:
Prof. LIU Tianfu
Fujian Institute of Research on the Structure of Matter
Chinese Academy of Sciences
Email: tfliu@fjirsm.ac.cn